Zinc transporter ZIP14 functions in hepatic zinc, iron and glucose homeostasis during the innate immune response (endotoxemia)
- PMID: 23110240
- PMCID: PMC3480510
- DOI: 10.1371/journal.pone.0048679
Zinc transporter ZIP14 functions in hepatic zinc, iron and glucose homeostasis during the innate immune response (endotoxemia)
Abstract
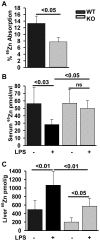
ZIP14 (slc39A14) is a zinc transporter induced in response to pro-inflammatory stimuli. ZIP14 induction accompanies the reduction in serum zinc (hypozincemia) of acute inflammation. ZIP14 can transport Zn(2+) and non-transferrin-bound Fe(2+) in vitro. Using a Zip14(-/-) mouse model we demonstrated that ZIP14 was essential for control of phosphatase PTP1B activity and phosphorylation of c-Met during liver regeneration. In the current studies, a global screening of ZIP transporter gene expression in response to LPS-induced endotoxemia was conducted. Following LPS, Zip14 was the most highly up-regulated Zip transcript in liver, but also in white adipose tissue and muscle. Using ZIP14(-/-) mice we show that ZIP14 contributes to zinc absorption from the gastrointestinal tract directly or indirectly as zinc absorption was decreased in the KOs. In contrast, Zip14(-/-) mice absorbed more iron. The Zip14 KO mice did not exhibit hypozincemia following LPS, but do have hypoferremia. Livers of Zip14-/- mice had increased transcript abundance for hepcidin, divalent metal transporter-1, ferritin and transferrin receptor-1 and greater accumulation of iron. The Zip14(-/-) phenotype included greater body fat, hypoglycemia and higher insulin levels, as well as increased liver glucose and greater phosphorylation of the insulin receptor and increased GLUT2, SREBP-1c and FASN expression. The Zip14 KO mice exhibited decreased circulating IL-6 with increased hepatic SOCS-3 following LPS, suggesting SOCS-3 inhibited insulin signaling which produced the hypoglycemia in this genotype. The results are consistent with ZIP14 ablation yielding abnormal labile zinc pools which lead to increased SOCS-3 production through G-coupled receptor activation and increased cAMP production as well as signaled by increased pSTAT3 via the IL-6 receptor, which inhibits IRS 1/2 phosphorylation. Our data show the role of ZIP14 in the hepatocyte is multi-functional since zinc and iron trafficking are altered in the Zip14(-/-) mice and their phenotype shows defects in glucose homeostasis.
Conflict of interest statement
Figures







References
-
- Lichten LA, Cousins RJ (2009) Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr 29: 153–176. - PubMed
-
- Cousins RJ, Lichten LA (2011) Zinc Transporters In: Rink L. ed. Zinc in Human Health. Amsterdam: IOS Press.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

