Pandemic influenza vaccine: characterization of A/California/07/2009 (H1N1) recombinant hemagglutinin protein and insights into H1N1 antigen stability
- PMID: 23110350
- PMCID: PMC3538074
- DOI: 10.1186/1472-6750-12-77
Pandemic influenza vaccine: characterization of A/California/07/2009 (H1N1) recombinant hemagglutinin protein and insights into H1N1 antigen stability
Abstract
Background: The recent H1N1 influenza pandemic illustrated the shortcomings of the vaccine manufacturing process. The A/California/07/2009 H1N1 pandemic influenza vaccine or A(H1N1)pdm09 was available late and in short supply as a result of delays in production caused by low yields and poor antigen stability. Recombinant technology offers the opportunity to shorten manufacturing time. A trivalent recombinant hemagglutinin (rHA) vaccine candidate for seasonal influenza produced using the baculovirus expression vector system (BEVS) was shown to be as effective and safe as egg-derived trivalent inactivated vaccine (TIV) in human clinical studies. In this study, we describe the characterization of the A/California/07/2009 rHA protein and compare the H1N1 pandemic rHA to other seasonal rHA proteins.
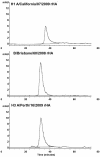
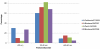
Results: Our data show that, like other rHA proteins, purified A/California/07/2009 rHA forms multimeric rosette-like particles of 20-40 nm that are biologically active and immunogenic in mice as assayed by hemagglutination inhibition (HAI) antibody titers. However, proteolytic digest analysis revealed that A/California/07/2009 rHA is more susceptible to proteolytic degradation than rHA proteins derived from other seasonal influenza viruses. We identified a specific proteolytic site conserved across multiple hemagglutinin (HA) proteins that is likely more accessible in A/California/07/2009 HA, possibly as a result of differences in its protein structure, and may contribute to lower antigen stability.
Conclusion: We conclude that, similar to the recombinant seasonal influenza vaccine, recombinant A(H1N1)pdm09 vaccine is likely to perform comparably to licensed A(H1N1)pdm09 vaccines and could offer manufacturing advantages.
Figures





References
-
- . President’s Council of Advisors on Science and Technology Report to the President on Reengineering the Influenza Vaccine Production Enterprise to Meet the Challenges of Pandemic Influenza. Washington, DC: Executive Office of the President; 2010. http://www.whitehouse.gov/sites/default/files/microsites/ostp/PCAST-Infl....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

