A link between interferon and augmented plasmin generation in exocrine gland damage in Sjögren's syndrome
- PMID: 23110742
- PMCID: PMC4259572
- DOI: 10.1016/j.jaut.2012.09.003
A link between interferon and augmented plasmin generation in exocrine gland damage in Sjögren's syndrome
Abstract
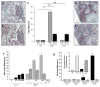
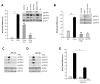
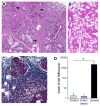
Sjögren's syndrome is an autoimmune disease that targets exocrine glands, but often exhibits systemic manifestations. Infiltration of the salivary and lacrimal glands by lymphoid and myeloid cells orchestrates a perpetuating immune response leading to exocrine gland damage and dysfunction. Th1 and Th17 lymphocyte populations and their products recruit additional lymphocytes, including B cells, but also large numbers of macrophages, which accumulate with disease progression. In addition to cytokines, chemokines, chitinases, and lipid mediators, macrophages contribute to a proteolytic milieu, underlying tissue destruction, inappropriate repair, and compromised glandular functions. Among the proteases enhanced in this local environment are matrix metalloproteases (MMP) and plasmin, generated by plasminogen activation, dependent upon plasminogen activators, such as tissue plasminogen activator (tPA). Not previously associated with salivary gland pathology, our evidence implicates enhanced tPA in the context of inflamed salivary glands revolving around lymphocyte-mediated activation of macrophages. Tracking down the mechanism of macrophage plasmin activation, the cytokines IFNγ and to a lesser extent, IFNα, via Janus kinase (JAK) and signal transducer and activator of transcription (STAT) activation, were found to be pivotal for driving the plasmin cascade of proteolytic events culminating in perpetuation of the inflammation and tissue damage, and suggesting intervention strategies to blunt irreversible tissue destruction.
Published by Elsevier Ltd.
Figures





References
-
- Voulgarelis M, Dafni UG, Isenberg DA, Moutsopoulos HM. Malignant lymphoma in primary Sjogren’s syndrome: a multicenter, retrospective, clinical study by the European Concerted Action on Sjogren’s Syndrome. Arthritis Rheum. 1999;42:1765–72. - PubMed
-
- Katsifis GE, Moutsopoulos NM, Wahl SM. T lymphocytes in Sjogren’s syndrome: contributors to and regulators of pathophysiology. Clin Rev Allergy Immunol. 2007;32:252–64. - PubMed
-
- Manoussakis MN, Boiu S, Korkolopoulou P, Kapsogeorgou EK, Kavantzas N, Ziakas P, et al. Rates of infiltration by macrophages and dendritic cells and expression of interleukin-18 and interleukin-12 in the chronic inflammatory lesions of Sjogren’s syndrome: correlation with certain features of immune hyperactivity and factors associated with high risk of lymphoma development. Arthritis Rheum. 2007;56:3977–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

