Stress-induced effects, which inhibit host defenses, alter leukocyte trafficking
- PMID: 23111563
- PMCID: PMC3631090
- DOI: 10.1007/s12192-012-0380-0
Stress-induced effects, which inhibit host defenses, alter leukocyte trafficking
Abstract
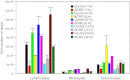
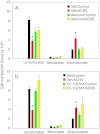
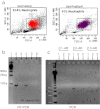
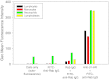
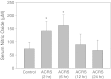
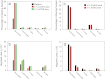
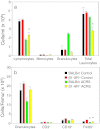
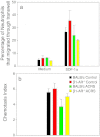
Acute cold restraint stress (ACRS) has been reported to suppress host defenses against Listeria monocytogenes, and this suppression was mediated by beta1-adrenoceptors (β1-ARs). Although ACRS appears to inhibit mainly early innate immune defenses, interference with leukocyte chemotaxis and the involvement of β1-AR (or β2-AR) signaling had not been assessed. Thus, the link between sympathetic nerve stimulation, release of neurotransmitters, and changes in blood leukocyte profiles, including oxidative changes, following ACRS was evaluated. The numbers of leukocyte subsets in the blood were differentially affected by β1-ARs and β2-ARs following ACRS; CD3(+) (CD4 and CD8) T-cells were shown to be decreased following ACRS, and the T cell lymphopenia was mediated mainly through a β2-AR mechanism, while the decrease in CD19(+) B-cells was influenced through both β1- and β2-ARs, as assessed by pharmacological and genetic manipulations. In contrast to the ACRS-induced loss of circulating lymphocytes, the number of circulating neutrophils was increased (i.e., neutrophilia), and this neutrophilia was mediated through β1-ARs. The increase in circulating neutrophils was not due to an increase in serum chemokines promoting neutrophil emigration from the bone marrow; rather it was due to neutrophil release from the bone marrow through activation of a β1-AR pathway. There was no loss of glutathione in any of the leukocyte subsets suggesting that there was minimal oxidative stress; however, there was early production of nitric oxide and generation of some protein radicals. Premature egress of neutrophils from bone marrow is suggested to be due to norepinephrine induction of nitric oxide, which affects the early release of neutrophils from bone marrow and lessens host defenses.
Figures



References
-
- Benschop RJ, Jacobs R, Sommer B, Schürmeyer TH, Raab JR, Schmidt RE, Schedlowski M. Modulation of the immunologic response to acute stress in humans by beta-blockade or benzodiazepines. FASEB J. 1996;10:517–524. - PubMed
-
- Bhardwaj V, Kanagawa O, Swanson PE, Unanue ER. Chronic Listeria infection in SCID mice: requirements for the carrier state and the dual role of T cells in transferring protection or suppression. J Immunol. 1998;160:376–384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

