The magnetite-based receptors in the beak of birds and their role in avian navigation
- PMID: 23111859
- PMCID: PMC3552369
- DOI: 10.1007/s00359-012-0769-3
The magnetite-based receptors in the beak of birds and their role in avian navigation
Abstract
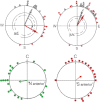
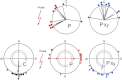
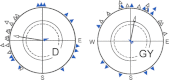
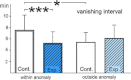
Iron-rich structures have been described in the beak of homing pigeons, chickens and several species of migratory birds and interpreted as magnetoreceptors. Here, we will briefly review findings associated with these receptors that throw light on their nature, their function and their role in avian navigation. Electrophysiological recordings from the ophthalmic nerve, behavioral studies and a ZENK-study indicate that the trigeminal system, the nerves innervating the beak, mediate information on magnetic changes, with the electrophysiological study suggesting that these are changes in intensity. Behavioral studies support the involvement of magnetite and the trigeminal system in magnetoreception, but clearly show that the inclination compass normally used by birds represents a separate system. However, if this compass is disrupted by certain light conditions, migrating birds show 'fixed direction' responses to the magnetic field, which originate in the receptors in the beak. Together, these findings point out that there are magnetite-based magnetoreceptors located in the upper beak close to the skin. Their natural function appears to be recording magnetic intensity and thus providing one component of the multi-factorial 'navigational map' of birds.
Figures

References
-
- Beason RC, Brennan WJ. Natural and induced magnetization in the Bobolink Dolichonyx oryzivorus (Aves: Icteridae) J Exp Biol. 1986;125:49–56.
-
- Beason RC, Nichols JE. Magnetic orientation and magnetically sensitive material in a transequatorial migratory bird. Nature. 1984;309:151–153. doi: 10.1038/309151a0. - DOI
-
- Beason RC, Semm P. Two different magnetic systems in avian orientation. In: Bell BD, Cossee RO, Flux JEC, Heather BD, Hitchmough RA, Robertson CJR, Williams MJ, editors. Acta XX Congr Int Ornithol. Wellington: New Zealand Ornithol Congr Trust Board; 1991. pp. 1813–1819.
-
- Beason RC, Semm P. Does the avian ophthalmic nerve carry magnetic information? J Exp Biol. 1996;199:1241–1244. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

