Identification of N-terminal residues of Sonic Hedgehog important for palmitoylation by Hedgehog acyltransferase
- PMID: 23112049
- PMCID: PMC3522284
- DOI: 10.1074/jbc.M112.426833
Identification of N-terminal residues of Sonic Hedgehog important for palmitoylation by Hedgehog acyltransferase
Abstract
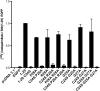
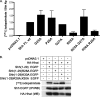
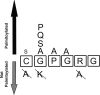
Sonic Hedgehog (Shh) is a secreted morphogen that regulates embryonic development. After removal of the signal peptide, Shh is processed to the mature, active form through autocleavage and a series of lipid modifications, including the attachment of palmitate. Covalent attachment of palmitate to the N-terminal cysteine of Shh is catalyzed by Hedgehog acyltransferase (Hhat) and is critical for proper signaling. The sequences within Shh that are responsible for palmitoylation by Hhat are not known. Here we show that the first six amino acids of mature Shh (CGPGRG) are sufficient for Hhat-mediated palmitoylation. Alanine scanning mutagenesis was used to determine the role of each amino acid and the positional sequence requirement in a cell-based Shh palmitoylation assay. Mutation of residues in the GPGR sequence to Ala had no effect on palmitoylation, provided that a positively charged residue was present within the first seven residues. The N-terminal position exhibited a strong but not exclusive requirement for Cys. Constructs with an N-terminal Ala were not palmitoylated. However, an N-terminal Ser served as a substrate for Hhat, but not the Drosophila melanogaster ortholog Rasp, highlighting a critical difference between the mammalian and fly enzymes. These findings define residues and regions within Shh that are necessary for its recognition as a substrate for Hhat-mediated palmitoylation. Finally, we report the results of a bioinformatics screen to identify other potential Hhat substrates encoded in the human genome.
Figures





Similar articles
-
Hhat is a palmitoylacyltransferase with specificity for N-palmitoylation of Sonic Hedgehog.J Biol Chem. 2008 Aug 8;283(32):22076-88. doi: 10.1074/jbc.M803901200. Epub 2008 Jun 4. J Biol Chem. 2008. PMID: 18534984 Free PMC article.
-
Identification of conserved regions and residues within Hedgehog acyltransferase critical for palmitoylation of Sonic Hedgehog.PLoS One. 2010 Jun 23;5(6):e11195. doi: 10.1371/journal.pone.0011195. PLoS One. 2010. PMID: 20585641 Free PMC article.
-
Membrane topology of hedgehog acyltransferase.J Biol Chem. 2015 Jan 23;290(4):2235-43. doi: 10.1074/jbc.M114.625764. Epub 2014 Dec 8. J Biol Chem. 2015. PMID: 25488661 Free PMC article.
-
Palmitoylation of Hedgehog proteins by Hedgehog acyltransferase: roles in signalling and disease.Open Biol. 2021 Mar;11(3):200414. doi: 10.1098/rsob.200414. Epub 2021 Mar 3. Open Biol. 2021. PMID: 33653085 Free PMC article. Review.
-
Palmitoylation of Hedgehog proteins.Vitam Horm. 2012;88:229-52. doi: 10.1016/B978-0-12-394622-5.00010-9. Vitam Horm. 2012. PMID: 22391306 Free PMC article. Review.
Cited by
-
Fatty acyl donor selectivity in membrane bound O-acyltransferases and communal cell fate decision-making.Biochem Soc Trans. 2015 Apr;43(2):235-9. doi: 10.1042/BST20140282. Biochem Soc Trans. 2015. PMID: 25849923 Free PMC article. Review.
-
Identification of the WNT1 residues required for palmitoylation by Porcupine.FEBS Lett. 2014 Dec 20;588(24):4815-24. doi: 10.1016/j.febslet.2014.11.016. Epub 2014 Nov 20. FEBS Lett. 2014. PMID: 25451226 Free PMC article.
-
A Residual N-Terminal Peptide Enhances Signaling of Depalmitoylated Hedgehog to the Patched Receptor.J Dev Biol. 2024 Apr 9;12(2):11. doi: 10.3390/jdb12020011. J Dev Biol. 2024. PMID: 38651456 Free PMC article.
-
Design, Synthesis, and Evaluation of Inhibitors of Hedgehog Acyltransferase.J Med Chem. 2024 Jan 25;67(2):1061-1078. doi: 10.1021/acs.jmedchem.3c01363. Epub 2024 Jan 10. J Med Chem. 2024. PMID: 38198226 Free PMC article.
-
Hedgehog acyltransferase as a target in pancreatic ductal adenocarcinoma.Oncogene. 2015 Jan 8;34(2):263-8. doi: 10.1038/onc.2013.575. Epub 2014 Jan 27. Oncogene. 2015. PMID: 24469057 Free PMC article.
References
-
- Ingham P. W., McMahon A. P. (2001) Hedgehog signaling in animal development. Paradigms and principles. Genes Dev. 15, 3059–3087 - PubMed
-
- Chiang C., Litingtung Y., Lee E., Young K. E., Corden J. L., Westphal H., Beachy P. A. (1996) Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 383, 407–413 - PubMed
-
- Roessler E., Belloni E., Gaudenz K., Jay P., Berta P., Scherer S. W., Tsui L. C., Muenke M. (1996) Mutations in the human Sonic Hedgehog gene cause holoprosencephaly. Nat. Genet. 14, 357–360 - PubMed
-
- Odent S., Atti-Bitach T., Blayau M., Mathieu M., Aug J., Delezo de A. L., Gall J. Y., Le Marec B., Munnich A., David V., Vekemans M. (1999) Expression of the Sonic hedgehog (SHH) gene during early human development and phenotypic expression of new mutations causing holoprosencephaly. Hum. Mol. Genet. 8, 1683–1689 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

