Immunoglobulin-like transcript receptors on human dermal CD14+ dendritic cells act as a CD8-antagonist to control cytotoxic T cell priming
- PMID: 23112154
- PMCID: PMC3503187
- DOI: 10.1073/pnas.1205785109
Immunoglobulin-like transcript receptors on human dermal CD14+ dendritic cells act as a CD8-antagonist to control cytotoxic T cell priming
Abstract
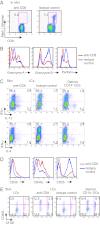
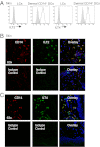
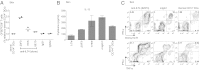
Human Langerhans cells (LCs) are highly efficient at priming cytolytic CD8(+) T cells compared with dermal CD14(+) dendritic cells (DCs). Here we show that dermal CD14(+) DCs instead prime a fraction of naïve CD8(+) T cells into cells sharing the properties of type 2 cytokine-secreting CD8(+) T cells (TC2). Differential expression of the CD8-antagonist receptors on dermal CD14(+) DCs, the Ig-like transcript (ILT) inhibitory receptors, explains the difference between the two types of DCs. Inhibition of CD8 function on LCs inhibited cytotoxic T lymphocytes (CTLs) and enhanced TC2 generation. In addition, blocking ILT2 or ILT4 on dermal CD14(+) DCs enhanced the generation of CTLs and inhibited TC2 cytokine production. Lastly, addition of soluble ILT2 and ILT4 receptors inhibited CTL priming by LCs. Thus, ILT receptor expression explains the polarization of CD8(+) T-cell responses by LCs vs. dermal CD14(+) DCs.
Conflict of interest statement
Conflict of interest statement: J.B., K.A.P., G.Z., and E.K. are co-inventors on a patent filing related to this work.
Figures


References
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. - PubMed
-
- Dudziak D, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315(5808):107–111. - PubMed
-
- Nestle FO, Zheng XG, Thompson CB, Turka LA, Nickoloff BJ. Characterization of dermal dendritic cells obtained from normal human skin reveals phenotypic and functionally distinctive subsets. J Immunol. 1993;151(11):6535–6545. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

