Interactions of subunit CCT3 in the yeast chaperonin CCT/TRiC with Q/N-rich proteins revealed by high-throughput microscopy analysis
- PMID: 23112166
- PMCID: PMC3503220
- DOI: 10.1073/pnas.1209277109
Interactions of subunit CCT3 in the yeast chaperonin CCT/TRiC with Q/N-rich proteins revealed by high-throughput microscopy analysis
Abstract
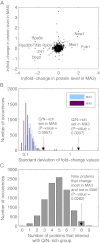
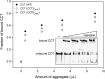
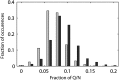
The eukaryotic chaperonin containing t-complex polypeptide 1 (CCT/TRiC) is an ATP-fueled machine that assists protein folding. It consists of two back-to-back stacked rings formed by eight different subunits that are arranged in a fixed permutation. The different subunits of CCT are believed to possess unique substrate binding specificities that are still mostly unknown. Here, we used high-throughput microscopy analysis of yeast cells to determine changes in protein levels and localization as a result of a Glu to Asp mutation in the ATP binding site of subunits 3 (CCT3) or 6 (CCT6). The mutation in subunit CCT3 was found to induce cytoplasmic foci termed P-bodies where mRNAs, which are not translated, accumulate and can be degraded. Analysis of the changes in protein levels and structural modeling indicate that P-body formation in cells with the mutation in CCT3 is linked to the specific interaction of this subunit with Gln/Asn-rich segments that are enriched in many P-body proteins. An in vitro gel-shift analysis was used to show that the mutation in subunit CCT3 interferes with the ability of CCT to bind a Gln/Asn-rich protein aggregate. More generally, the strategy used in this work can be used to unravel the substrate specificities of other chaperone systems.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Horwich AL, Fenton WA, Chapman E, Farr GW. Two families of chaperonin: physiology and mechanism. Annu Rev Cell Dev Biol. 2007;23:115–145. - PubMed
-
- Yébenes H, Mesa P, Muñoz IG, Montoya G, Valpuesta JM. Chaperonins: two rings for folding. Trends Biochem Sci. 2011;36(8):424–432. - PubMed
-
- Azia A, Unger R, Horovitz A. What distinguishes GroEL substrates from other Escherichia coli proteins? FEBS J. 2012;279(4):543–550. - PubMed
-
- Gao Y, Thomas JO, Chow RL, Lee GH, Cowan NJ. A cytoplasmic chaperonin that catalyzes β-actin folding. Cell. 1992;69(6):1043–1050. - PubMed
-
- Yaffe MB, et al. TCP1 complex is a molecular chaperone in tubulin biogenesis. Nature. 1992;358(6383):245–248. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

