Glial progenitor cell-based treatment and modeling of neurological disease
- PMID: 23112326
- PMCID: PMC3548656
- DOI: 10.1126/science.1218071
Glial progenitor cell-based treatment and modeling of neurological disease
Abstract
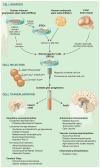
The diseases of myelin are among the most prevalent and disabling conditions in neurology. These diseases include both the vascular and inflammatory demyelinating disorders of adulthood, as well as the childhood leukodystrophies and cerebral palsy. These fundamentally glial disorders may be amenable to treatment by glial progenitor cells (GPCs), which give rise to astroglia and myelin-producing oligodendrocytes. Given the development of new methods for generating and isolating human GPCs, the myelin disorders may now be compelling targets for cell-based therapy. In addition, the efficient engraftment and expansion of human GPCs in murine hosts has led to the development of human glial chimeric mouse brains, which provides new opportunities for studying the species-specific roles of human glia in cognition, as well as in disease pathogenesis.
Figures

References
-
- Roy N, Windrem M, Goldman SA. In: Myelin Biology and Disorders. Lazzarini R, editor. Elsevier; Amsterdam: 2004. pp. 259–287.
-
- Tkachev D, et al. Lancet. 2003;362:798. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

