Inhalational system for Etoposide liposomes: formulation development and in vitro deposition
- PMID: 23112400
- PMCID: PMC3480751
- DOI: 10.4103/0250-474X.100240
Inhalational system for Etoposide liposomes: formulation development and in vitro deposition
Abstract
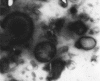
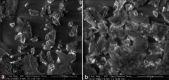
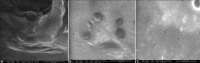
Etoposide is a semisynthetic compound, widely used in treatment of non small cell lung cancer. However, frequent dosing and adverse effects remain a major concern in the use of etoposide. Liposomal systems for pulmonary drug delivery have been particularly attractive because of their compatibility with lung surfactant components. In the present investigation, pulmonary liposomal delivery system of etoposide was prepared by film hydration method. Various parameters were optimized with respect to entrapment efficiency as well as particle size of etoposide liposomes. For better shelf life of etoposide liposomes, freeze drying using trehalose as cryoprotectant was carried out. The liposomes were characterized for entrapment efficiency, particle size, surface topography, and in vitro drug release was carried out in simulated lung fluid at 37° at pH 7.4. The respirable or fine particle fraction was determined by using twin stage impinger. The stability study of freeze dried as well as aqueous liposomal systems was carried out at 2-8° and at ambient temperature (28±4°). The freeze dried liposomes showed better fine particle fraction and drug content over the period of six months at ambient as well as at 2-8° storage condition compared to aqueous dispersion of liposomes.
Keywords: Etoposide; inhalation; liposome; pulmonary delivery; twin stage impinger.
Figures
Similar articles
-
Development and evaluation of inhalational liposomal system of budesonide for better management of asthma.Indian J Pharm Sci. 2010 Jul;72(4):442-8. doi: 10.4103/0250-474X.73916. Indian J Pharm Sci. 2010. PMID: 21218054 Free PMC article.
-
Development of Chitosan-based Dry Powder Inhalation System of Cisplatin for Lung Cancer.Indian J Pharm Sci. 2012 Nov;74(6):521-6. doi: 10.4103/0250-474X.110584. Indian J Pharm Sci. 2012. PMID: 23798777 Free PMC article.
-
Liposomal budesonide for dry powder inhaler: preparation and stabilization.AAPS PharmSciTech. 2001 Nov 30;2(4):25. doi: 10.1208/pt020425. AAPS PharmSciTech. 2001. PMID: 14727862 Free PMC article.
-
Formulation optimization of etoposide loaded PLGA nanoparticles by double factorial design and their evaluation.Curr Drug Deliv. 2010 Jan;7(1):51-64. doi: 10.2174/156720110790396517. Curr Drug Deliv. 2010. PMID: 20044908 Review.
-
Recent advances in liposomal dry powder formulations: preparation and evaluation.Expert Opin Drug Deliv. 2009 Jan;6(1):71-89. doi: 10.1517/17425240802652309. Expert Opin Drug Deliv. 2009. PMID: 19236209 Review.
Cited by
-
Development of an Inhaled Sustained Release Dry Powder Formulation of Salbutamol Sulphate, an Antiasthmatic Drug.Indian J Pharm Sci. 2016 Jan-Feb;78(1):136-42. doi: 10.4103/0250-474x.180261. Indian J Pharm Sci. 2016. PMID: 27168692 Free PMC article.
-
Folic Acid-Targeted Etoposide Cubosomes for Theranostic Application of Cancer Cell Imaging and Therapy.Med Sci Monit. 2017 May 22;23:2426-2435. doi: 10.12659/msm.904683. Med Sci Monit. 2017. PMID: 28529305 Free PMC article.
-
Pharmaceutical and pharmacological approaches for bioavailability enhancement of etoposide.J Biosci. 2014 Mar;39(1):139-44. doi: 10.1007/s12038-013-9399-3. J Biosci. 2014. PMID: 24499798 Review.
References
-
- Courrier HM, Butz N, Vandamme TF. Pulmonary drug delivery systems: Recent developments and prospects. Crit Rev Ther Drug Carrier Syst. 2002;19:425–98. - PubMed
-
- Groneberg DA, Witt C, Wagner U, Chung KF, Fischer A. Fundamentals of pulmonary drug delivery. Respir Med. 2003;97:382–7. - PubMed
-
- Zeng XM, Martin GP, Marriott C. The controlled delivery of drugs to the lung. Int J Pharm. 1995;124:149–64.
-
- Witek TJ. The Fate of Inhaled Drugs: The pharmacokinetics and pharmacodynamics of drugs administered by aerosol. Respir Care. 2000;45:826–30. - PubMed
-
- Clark AR. Pulmonary Delivery Technology: Recent Advances and Potential for the New Millennium. In: Hickey AJ, editor. Pharmaceutical Inhalation Aerosol Technology. 2nd ed. New York: Marcel Dekker Inc; 2004. pp. 563–84.
LinkOut - more resources
Full Text Sources
