Lab-on-a-chip pathogen sensors for food safety
- PMID: 23112625
- PMCID: PMC3472853
- DOI: 10.3390/s120810713
Lab-on-a-chip pathogen sensors for food safety
Abstract
There have been a number of cases of foodborne illness among humans that are caused by pathogens such as Escherichia coli O157:H7, Salmonella typhimurium, etc. The current practices to detect such pathogenic agents are cell culturing, immunoassays, or polymerase chain reactions (PCRs). These methods are essentially laboratory-based methods that are not at all real-time and thus unavailable for early-monitoring of such pathogens. They are also very difficult to implement in the field. Lab-on-a-chip biosensors, however, have a strong potential to be used in the field since they can be miniaturized and automated; they are also potentially fast and very sensitive. These lab-on-a-chip biosensors can detect pathogens in farms, packaging/processing facilities, delivery/distribution systems, and at the consumer level. There are still several issues to be resolved before applying these lab-on-a-chip sensors to field applications, including the pre-treatment of a sample, proper storage of reagents, full integration into a battery-powered system, and demonstration of very high sensitivity, which are addressed in this review article. Several different types of lab-on-a-chip biosensors, including immunoassay- and PCR-based, have been developed and tested for detecting foodborne pathogens. Their assay performance, including detection limit and assay time, are also summarized. Finally, the use of optical fibers or optical waveguide is discussed as a means to improve the portability and sensitivity of lab-on-a-chip pathogen sensors.
Keywords: E. coli; Salmonella; bioMEMS; food safety; microfluidics; water safety.
Figures
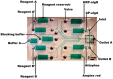
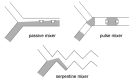
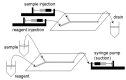


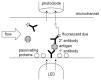
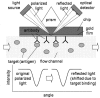

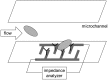
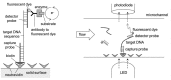
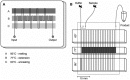

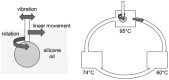
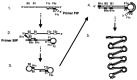
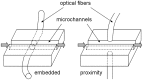

References
-
- McCabe-Sellers B.J., Beattie S.E. Food safety: Emerging trends in foodborne illness surveillance and prevention. J. Am. Dietetic Assoc. 2004;104:1708–1717. - PubMed
-
- Horby P.W., O'Brien S.J., Adak G.K., Graham C., Hawker J.I., Hunter P., Lane C., Lawson A.J., Mitchell R.T., Reacher M.H. A national outbreak of multi-resistant Salmonella enteric serovar typhimurium definitive phage type (DT) 104 associated with consumption of lettuce. Epidemiol. Infect. 2003;130:169–178. - PMC - PubMed
-
- United States Department of Agriculture Economic Research Services . Foodborne Illness Cost Calculator. USDA; Washington, DC, USA: 2010.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

