CD2 promotes human natural killer cell membrane nanotube formation
- PMID: 23112830
- PMCID: PMC3480409
- DOI: 10.1371/journal.pone.0047664
CD2 promotes human natural killer cell membrane nanotube formation
Abstract
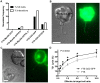
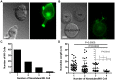
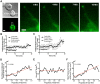
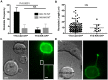
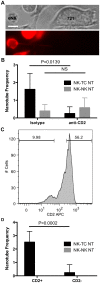
Membrane nanotubes are thin membranous projections that physically connect two cells. While nanotubes have been studied in human natural killer (NK) cells and are implicated in aiding NK cell cytotoxic function, requirements for their formation to susceptible target cells remain incompletely understood. Here we demonstrate that the CD2-CD58/48 receptor-ligand interaction promotes and is required for nanotube formation in human NK cells. In the CD2(-) NK cell line YTS, a stable CD2 expression variant enabled effective nanotube formation, and was associated with better cytotoxic function. Importantly, only interactions between an NK cell and a susceptible target cell were associated with multiple nanotubes and the number of nanotubes was inversely correlated with their length. Quantitative live cell fluorescence microscopy of CD2 nanotubes revealed time-dependent enrichment and localization of CD2 to the nanotube tip, and blocking CD2 receptor-ligand interactions prevented nanotube formation. Increased nanotube formation was not simply a feature of receptor-ligand pairing, as a KIR-MHC interaction in the same cell line system failed to promote nanotube formation. Additionally, blocking LFA-1-ICAM and 2B4-CD48 receptor-ligand interactions failed to inhibit nanotube formation. Thus only specific receptor-ligand pairs promote nanotubes. CD2 also promoted nanotube formation in ex vivo NK cells suggesting that CD2 plays a crucial role in the generation of nanotubes between an NK cell and its target.
Conflict of interest statement
Figures
Similar articles
-
CD58 Immunobiology at a Glance.Front Immunol. 2021 Jun 8;12:705260. doi: 10.3389/fimmu.2021.705260. eCollection 2021. Front Immunol. 2021. PMID: 34168659 Free PMC article. Review.
-
Homotypic cell to cell cross-talk among human natural killer cells reveals differential and overlapping roles of 2B4 and CD2.J Biol Chem. 2010 Dec 31;285(53):41755-64. doi: 10.1074/jbc.M110.137976. Epub 2010 Sep 2. J Biol Chem. 2010. PMID: 20813844 Free PMC article.
-
Membrane nanotubes facilitate long-distance interactions between natural killer cells and target cells.Proc Natl Acad Sci U S A. 2010 Mar 23;107(12):5545-50. doi: 10.1073/pnas.0910074107. Epub 2010 Mar 8. Proc Natl Acad Sci U S A. 2010. PMID: 20212116 Free PMC article.
-
Natural killer cell cytotoxicity of breast cancer targets is enhanced by two distinct mechanisms of antibody-dependent cellular cytotoxicity against LFA-3 and HER2/neu.Exp Hematol. 1999 Oct;27(10):1533-41. doi: 10.1016/s0301-472x(99)00089-2. Exp Hematol. 1999. PMID: 10517495
-
2B4 (CD244) and CS1: novel members of the CD2 subset of the immunoglobulin superfamily molecules expressed on natural killer cells and other leukocytes.Immunol Rev. 2001 Jun;181:234-49. doi: 10.1034/j.1600-065x.2001.1810120.x. Immunol Rev. 2001. PMID: 11513145 Review.
Cited by
-
CD58 Immunobiology at a Glance.Front Immunol. 2021 Jun 8;12:705260. doi: 10.3389/fimmu.2021.705260. eCollection 2021. Front Immunol. 2021. PMID: 34168659 Free PMC article. Review.
-
Practical NK cell phenotyping and variability in healthy adults.Immunol Res. 2015 Jul;62(3):341-56. doi: 10.1007/s12026-015-8664-y. Immunol Res. 2015. PMID: 26013798 Free PMC article.
-
Use of Alefacept for Preconditioning in Multiply Transfused Pediatric Patients with Nonmalignant Diseases.Biol Blood Marrow Transplant. 2015 Oct;21(10):1845-52. doi: 10.1016/j.bbmt.2015.06.005. Epub 2015 Jun 19. Biol Blood Marrow Transplant. 2015. PMID: 26095669 Free PMC article. Clinical Trial.
-
Tunneling nanotube (TNT)-mediated neuron-to neuron transfer of pathological Tau protein assemblies.Acta Neuropathol Commun. 2016 Nov 4;4(1):117. doi: 10.1186/s40478-016-0386-4. Acta Neuropathol Commun. 2016. PMID: 27809932 Free PMC article.
-
Six-Gene Signature Associated with Immune Cells in the Progression of Atherosclerosis Discovered by Comprehensive Bioinformatics Analyses.Cardiovasc Ther. 2020 Jul 25;2020:1230513. doi: 10.1155/2020/1230513. eCollection 2020. Cardiovasc Ther. 2020. PMID: 32821283 Free PMC article.
References
-
- Davis DM (2009) Mechanisms and functions for the duration of intercellular contacts made by lymphocytes. Nature reviews Immunology 9: 543–555. - PubMed
-
- McNerney ME, Kumar V (2006) The CD2 family of natural killer cell receptors. Current topics in microbiology and immunology 298: 91–120. - PubMed
-
- Barber DF, Long EO (2003) Coexpression of CD58 or CD48 with intercellular adhesion molecule 1 on target cells enhances adhesion of resting NK cells. Journal of immunology 170: 294–299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

