Regulatory roles of cytokinins and cytokinin signaling in response to potassium deficiency in Arabidopsis
- PMID: 23112848
- PMCID: PMC3480408
- DOI: 10.1371/journal.pone.0047797
Regulatory roles of cytokinins and cytokinin signaling in response to potassium deficiency in Arabidopsis
Abstract
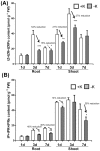
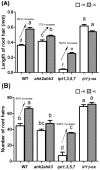
Potassium (K) is an important plant macronutrient that has various functions throughout the whole plant over its entire life span. Cytokinins (CKs) are known to regulate macronutrient homeostasis by controlling the expression of nitrate, phosphate and sulfate transporters. Although several studies have described how CKs signal deficiencies for some macronutrients, the roles of CKs in K signaling are poorly understood. CK content has been shown to decrease under K-starved conditions. Specifically, a CK-deficient mutant was more tolerant to low K than wild-type; however, a plant with an overaccumulation of CKs was more sensitive to low K. These results suggest that K deprivation alters CK metabolism, leading to a decrease in CK content. To investigate this phenomenon further, several Arabidopsis lines, including a CK-deficient mutant and CK receptor mutants, were analyzed in low K conditions using molecular, genetic and biochemical approaches. ROS accumulation and root hair growth in low K were also influenced by CKs. CK receptor mutants lost the responsiveness to K-deficient signaling, including ROS accumulation and root hair growth, but the CK-deficient mutant accumulated more ROS and exhibited up-regulated expression of HAK5, which is a high-affinity K uptake transporter gene that is rapidly induced by low K stress in ROS- and ethylene-dependent manner in response to low K. From these results, we conclude that a reduction in CK levels subsequently allows fast and effective stimulation of low K-induced ROS accumulation, root hair growth and HAK5 expression, leading to plant adaptation to low K conditions.
Conflict of interest statement
Figures




Similar articles
-
Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis.Plant Cell. 2011 Jun;23(6):2169-83. doi: 10.1105/tpc.111.087395. Epub 2011 Jun 30. Plant Cell. 2011. PMID: 21719693 Free PMC article.
-
The role of cytokinin in selenium stress response in Arabidopsis.Plant Sci. 2019 Apr;281:122-132. doi: 10.1016/j.plantsci.2019.01.028. Epub 2019 Feb 4. Plant Sci. 2019. PMID: 30824045
-
Ethylene mediates response and tolerance to potassium deprivation in Arabidopsis.Plant Cell. 2009 Feb;21(2):607-21. doi: 10.1105/tpc.108.063099. Epub 2009 Feb 3. Plant Cell. 2009. PMID: 19190240 Free PMC article.
-
AZGs: a new family of cytokinin transporters.Biochem Soc Trans. 2024 Aug 28;52(4):1841-1848. doi: 10.1042/BST20231537. Biochem Soc Trans. 2024. PMID: 38979638 Review.
-
Regulation of potassium transport and signaling in plants.Curr Opin Plant Biol. 2017 Oct;39:123-128. doi: 10.1016/j.pbi.2017.06.006. Epub 2017 Jul 13. Curr Opin Plant Biol. 2017. PMID: 28710919 Review.
Cited by
-
Cytokinin-regulated targets of Cytokinin Response Factor 6 are involved in potassium transport.Plant Direct. 2020 Dec 8;4(12):e00291. doi: 10.1002/pld3.291. eCollection 2020 Dec. Plant Direct. 2020. PMID: 36406052 Free PMC article.
-
Root engineering in maize by increasing cytokinin degradation causes enhanced root growth and leaf mineral enrichment.Plant Mol Biol. 2021 Aug;106(6):555-567. doi: 10.1007/s11103-021-01173-5. Epub 2021 Jul 17. Plant Mol Biol. 2021. PMID: 34275101 Free PMC article.
-
Hormonal control of sulfate uptake and assimilation.Plant Mol Biol. 2016 Aug;91(6):617-27. doi: 10.1007/s11103-016-0438-y. Epub 2016 Jan 25. Plant Mol Biol. 2016. PMID: 26810064 Review.
-
Nitrogen Supplementation Modulates Morphological, Biochemical, Yield and Quality Attributes of Peppermint.Plants (Basel). 2023 Feb 10;12(4):809. doi: 10.3390/plants12040809. Plants (Basel). 2023. PMID: 36840157 Free PMC article.
-
Induction of Barley Silicon Transporter HvLsi1 and HvLsi2, increased silicon concentration in the shoot and regulated Starch and ABA Homeostasis under Osmotic stress and Concomitant Potassium Deficiency.Front Plant Sci. 2017 Aug 3;8:1359. doi: 10.3389/fpls.2017.01359. eCollection 2017. Front Plant Sci. 2017. PMID: 28824688 Free PMC article.
References
-
- Lopez-Bucio J, Cruz-Ramirez A, Herrera-Estrella L (2003) The role of nutrient availability in regulating root architecture. Curr Opin Plant Biol 6: 280–287. - PubMed
-
- Schachtman DP, Shin R (2007) Nutrient sensing and signaling: NPKS. Annu Rev Plant Biol 58: 47–69. - PubMed
-
- Amtmann J, Amtmann K (2006) Strength training for EMS professionals: fit to respond. J Emergency Med Services 31: 52–56. - PubMed
-
- Leigh RA, Jones RGW (1984) A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell. New phytol 97: 1–13.
-
- Marschner H (1995) Minera nutrient of higher plants. Academic Press, London 2nd Edn.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

