Parasite burden in hamsters infected with two different strains of leishmania (Leishmania) infantum: "Leishman Donovan units" versus real-time PCR
- PMID: 23112869
- PMCID: PMC3480442
- DOI: 10.1371/journal.pone.0047907
Parasite burden in hamsters infected with two different strains of leishmania (Leishmania) infantum: "Leishman Donovan units" versus real-time PCR
Abstract
To develop and test new therapeutics and immune prophylaxis strategies for visceral leishmaniasis (VL), understanding tissue parasitism evolution after experimental infection with Leishmania infantum is important. Experimental infection in a hamster model (Mesocricetus auratus) reproduces several typical aspects of canine and human VL that are closely related to the inoculum's route. We quantified the parasitism in the liver and spleen of hamsters experimentally infected by various routes (intradermal, intraperitoneal, and intracardiac [IC]) and different strains of L. infantum (MHOM/BR/74/PP75 and Wild) and compared two different methodologies to evaluate tissue parasitism (Leishman Donovan units [LDU] and real-time qPCR). In addition, the quantification of specific total-IgG in the serum of uninfected and infected hamsters was determined by ELISA. The animals were followed for 1, 3, 6 and 9 months post-infection for survival analysis. We found that infection with the Wild strain by the IC route resulted in higher mortality. Positive antibody (IgG) responses were detected with higher peaks at 6 and 9 months in the IC group inoculated with PP75 strain. However, in animals infected with the Wild strain the IgG levels were elevated in all infected groups during all the time evaluated. We also observed by LDU analysis that the IC route lead to higher parasitism in the liver and spleen with both strains. Furthermore, qPCR showed higher sensitivity for identifying animals with low parasitic burden. In conclusion, qPCR can be useful for assessing parasitism in the spleen and liver of a hamster model infected with L. infantum independent of the route of infection, and this technique may become an essential tool for assessing parasite density in the hamster model after experimental treatment or immunization with potential vaccine candidates.
Conflict of interest statement
Figures
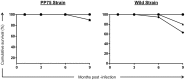
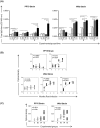
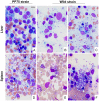
Similar articles
-
Clinical, hematological and biochemical alterations in hamster (Mesocricetus auratus) experimentally infected with Leishmania infantum through different routes of inoculation.Parasit Vectors. 2016 Mar 31;9:181. doi: 10.1186/s13071-016-1464-y. Parasit Vectors. 2016. PMID: 27030128 Free PMC article.
-
In Vivo Imaging of Leishmania infantum-infected Hamsters by Gingival Inoculation of Axenic Amastigotes Expressing Luciferase.J Vis Exp. 2025 Apr 4;(218). doi: 10.3791/67050. J Vis Exp. 2025. PMID: 40257961
-
Verification and monitoring of visceral leishmaniasis in hamsters caused by Leishmania infantum, using non-invasive approaches involving ultrasound imaging and blood gases.Exp Parasitol. 2019 Jun;201:78-89. doi: 10.1016/j.exppara.2019.04.012. Epub 2019 Apr 29. Exp Parasitol. 2019. PMID: 31047987
-
Mechanisms of resistance and susceptibility to experimental visceral leishmaniosis: BALB/c mouse versus Syrian hamster model.Vet Res. 2011 Feb 23;42(1):39. doi: 10.1186/1297-9716-42-39. Vet Res. 2011. PMID: 21345200 Free PMC article. Review.
-
Regulation of immunity during visceral Leishmania infection.Parasit Vectors. 2016 Mar 1;9:118. doi: 10.1186/s13071-016-1412-x. Parasit Vectors. 2016. PMID: 26932389 Free PMC article. Review.
Cited by
-
Leishmania infantum infection does not affect the main composition of the intestinal microbiome of the Syrian hamster.Parasit Vectors. 2022 Dec 15;15(1):468. doi: 10.1186/s13071-022-05576-1. Parasit Vectors. 2022. PMID: 36522762 Free PMC article.
-
Combination of liposomal CpG oligodeoxynucleotide 2006 and miltefosine induces strong cell-mediated immunity during experimental visceral leishmaniasis.PLoS One. 2014 Apr 14;9(4):e94596. doi: 10.1371/journal.pone.0094596. eCollection 2014. PLoS One. 2014. PMID: 24732039 Free PMC article.
-
Next-Generation Leishmanization: Revisiting Molecular Targets for Selecting Genetically Engineered Live-Attenuated Leishmania.Microorganisms. 2023 Apr 16;11(4):1043. doi: 10.3390/microorganisms11041043. Microorganisms. 2023. PMID: 37110466 Free PMC article. Review.
-
Performance of LBSap vaccine after intradermal challenge with L. infantum and saliva of Lu. longipalpis: immunogenicity and parasitological evaluation.PLoS One. 2012;7(11):e49780. doi: 10.1371/journal.pone.0049780. Epub 2012 Nov 26. PLoS One. 2012. PMID: 23189161 Free PMC article.
-
Vaccination with Formulation of Nanoparticles Loaded with Leishmania amazonensis Antigens Confers Protection against Experimental Visceral Leishmaniasis in Hamster.Vaccines (Basel). 2023 Jan 2;11(1):111. doi: 10.3390/vaccines11010111. Vaccines (Basel). 2023. PMID: 36679956 Free PMC article.
References
-
- WHO (2010) Working to overcome the global impact of neglected tropical diseases. First WHO reported on neglected tropical diseases. In WHO Geneva 184.
-
- Desjeux P (2004) Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis 27: 305–318. - PubMed
-
- Hommel M, Jaffe CL, Travi B, Milon G (1995) Experimental models for leishmaniasis and for testing anti-leishmanial vaccines. Ann Trop Med Parasitol 89 Suppl 155–73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

