Phase II trial of sorafenib in patients with advanced anaplastic carcinoma of the thyroid
- PMID: 23113752
- PMCID: PMC3643255
- DOI: 10.1089/thy.2012.0103
Phase II trial of sorafenib in patients with advanced anaplastic carcinoma of the thyroid
Abstract
Background: Anaplastic thyroid cancer (ATC) is a rare but highly aggressive malignancy with a median survival of 3-5 months. The BRAF oncogene is mutated to its active form in up to 24% of ATC cases. Sorafenib is a tyrosine kinase inhibitor that acts on the RAF-1 serine/threonine kinase. In preclinical mouse models, sorafenib inhibits the growth of ATC xenografts and improves survival. No study of sorafenib in ATC has been conducted. We conducted a multi-institutional phase II trial of sorafenib in patients with ATC who had failed up to two previous therapies.
Methods: The primary endpoint of the trial was the Response Evaluation Criteria In Solid Tumors (RECIST)-defined imaging response rate. Twenty patients with ATC were treated with sorafenib 400 mg twice daily.
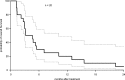
Results: Two of the 20 patients had a partial response (10%) and an additional 5 of 20 (25%) had stable disease. The duration of response in the two responders was 10 and 27 months, respectively. For the patients with stable disease, the median duration was 4 months (range 3-11 months). The overall median progression-free survival was 1.9 months with a median and a 1-year survival of 3.9 months and 20%, respectively. Toxicity was manageable and as previously described for sorafenib, including hypertension and skin rash.
Conclusion: Sorafenib has activity in ATC, but at a low frequency and similar to our previous experience with fosbretabulin. One patient with a response had previously progressed on fosbretabulin. Toxicities were both predictable and manageable.
Trial registration: ClinicalTrials.gov NCT00126568.
Figures
References
-
- Davies L. Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. - PubMed
-
- Ain KB. Anaplastic thyroid carcinoma: a therapeutic challenge. Semin Surg Oncol. 1999;16:64–69. - PubMed
-
- Edge SB. Byrd DR. Compton CC. Fritz AG. Greene FL. In: AJCC Cancer Staging Manual. 7th. Trotti A, editor. New York, NY: Springer Verlag; 2010.
-
- Kim JH. Leeper RD. Treatment of locally advanced thyroid carcinoma with combination doxorubicin and radiation therapy. Cancer. 1987;60:2372–2375. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous