Macropinocytosis is responsible for the uptake of pathogenic and non-pathogenic mycobacteria by B lymphocytes (Raji cells)
- PMID: 23113903
- PMCID: PMC3559283
- DOI: 10.1186/1471-2180-12-246
Macropinocytosis is responsible for the uptake of pathogenic and non-pathogenic mycobacteria by B lymphocytes (Raji cells)
Abstract
Background: The classical roles of B cells include the production of antibodies and cytokines and the generation of immunological memory, these being key factors in the adaptive immune response. However, their role in innate immunity is currently being recognised. Traditionally, B cells have been considered non-phagocytic cells; therefore, the uptake of bacteria by B cells is not extensively documented. In this study, we analysed some of the features of non-specific bacterial uptake by B lymphocytes from the Raji cell line. In our model, B cells were infected with Mycobacterium tuberculosis (MTB), Mycobacterium smegmatis (MSM), and Salmonella typhimurium (ST).
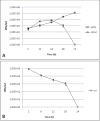
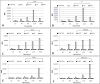
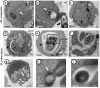
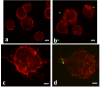
Results: Our observations revealed that the Raji B cells were readily infected by the three bacteria that were studied. All of the infections induced changes in the cellular membrane during bacterial internalisation. M. smegmatis and S. typhimurium were able to induce important membrane changes that were characterised by abundant filopodia and lamellipodia formation. These membrane changes were driven by actin cytoskeletal rearrangements. The intracellular growth of these bacteria was also controlled by B cells. M. tuberculosis infection also induced actin rearrangement-driven membrane changes; however, the B cells were not able to control this infection. The phorbol 12-myristate 13-acetate (PMA) treatment of B cells induced filopodia and lamellipodia formation, the production of spacious vacuoles (macropinosomes), and the fluid-phase uptake that is characteristic of macropinocytosis. S. typhimurium infection induced the highest fluid-phase uptake, although both mycobacteria also induced fluid uptake. A macropinocytosis inhibitor such as amiloride was used and abolished the bacterial uptake and the fluid-phase uptake that is triggered during the bacterial infection.
Conclusions: Raji B cells can internalise S. typhimurium and mycobacteria through an active process, such as macropinocytosis, although the resolution of the infection depends on factors that are inherent in the virulence of each pathogen.
Figures



References
-
- Lund FE, Garvy BA, Randall TD, Harris DP. Regulatory roles for cytokine-producing B cells in infection and autoimmune disease. Curr Dir Autoimmun. 2005;8:25–54. - PubMed
-
- Putnam MA, Moquin AE, Merrihew M, Outcalt C, Sorge E, Caballero A, Gondré-Lewis TA, Drake JR. Lipid raft-independent B cell receptor-mediated antigen internalization and intracellular trafficking. J Immunol. 2003;170:905–912. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

