Pyronaridine-artesunate granules versus artemether-lumefantrine crushed tablets in children with Plasmodium falciparum malaria: a randomized controlled trial
- PMID: 23113947
- PMCID: PMC3566922
- DOI: 10.1186/1475-2875-11-364
Pyronaridine-artesunate granules versus artemether-lumefantrine crushed tablets in children with Plasmodium falciparum malaria: a randomized controlled trial
Abstract
Background: Children are most vulnerable to malaria. A pyronaridine-artesunate pediatric granule formulation is being developed for the treatment of uncomplicated Plasmodium falciparum malaria.
Methods: This phase III, multi-center, comparative, open-label, parallel-group, controlled clinical trial included patients aged ≤12 years, bodyweight ≥5 to <25 kg, with a reported history of fever at inclusion or in the previous 24 h and microscopically-confirmed uncomplicated P. falciparum malaria. Patients were randomized (2:1) to pyronaridine-artesunate granules (60/20 mg) once daily or artemether-lumefantrine crushed tablets (20/120 mg) twice daily, both dosed by bodyweight, orally (liquid suspension) for three days.
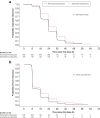
Results: Of 535 patients randomized, 355 received pyronaridine-artesunate and 180 received artemether-lumefantrine. Day-28 adequate clinical and parasitological response (ACPR), corrected for re-infection using polymerase chain reaction (PCR) genotyping (per-protocol population) was 97.1% (329/339; 95% CI 94.6, 98.6) for pyronaridine-artesunate; 98.8% (165/167; 95% CI 95.7, 99.9) for artemether-lumefantrine. The primary endpoint was achieved: pyronaridine-artesunate PCR-corrected day-28 ACPR was statistically significantly >90% (P < .0001). Pyronaridine-artesunate was non-inferior to artemether-lumefantrine: treatment difference -1.8% (95% CI -4.3 to 1.6). The incidence of drug-related adverse events was 37.2% (132/355) with pyronaridine-artesunate, 44.4% (80/180) with artemether-lumefantrine. Clinical biochemistry results showed similar mean changes versus baseline in the two treatment groups. From day 3 until study completion, one patient in each treatment group had peak alanine aminotransferase (ALT) >3 times the upper limit of normal (ULN) and peak total bilirubin >2xULN (i.e. within the Hy's law definition).
Conclusions: The pyronaridine-artesunate pediatric granule formulation was efficacious and was non-inferior to artemether-lumefantrine. The adverse event profile was similar for the two comparators. Pyronaridine-artesunate should be considered for inclusion in paediatric malaria treatment programmes.
Trial registration: ClinicalTrials.gov: identifier NCT00541385.
Figures


References
-
- Roll Back Malaria Partnership Secretariat. World malaria Day 2010. Africa Update. http://www.rollbackmalaria.org/ProgressImpactSeries/docs/wmd2010report-e....
-
- World Health Organization. World malaria report 2011. http://www.who.int/malaria/world_malaria_report_2011/9789241564403_eng.pdf.
-
- World Health Organization. Guidelines for the treatment of malaria. second. http://whqlibdoc.who.int/publications/2010/9789241547925_eng.pdf.
-
- World Health Organization. WHO list of prequalified medicinal products. http://apps.who.int/prequal/query/ProductRegistry.aspx?list=ma.
-
- Tshefu AK, Gaye O, Kayentao K, Thompson R, Bhatt KM, Sesay SS, Bustos DG, Tjitra E, Bedu-Addo G, Borghini-Fuhrer I, Duparc S, Shin CS, Fleckenstein L. Efficacy and safety of a fixed-dose oral combination of pyronaridine-artesunate compared with artemether-lumefantrine in children and adults with uncomplicated Plasmodium falciparum malaria: a randomised non-inferiority trial. Lancet. 2010;375:1457–1467. doi: 10.1016/S0140-6736(10)60322-4. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

