Role of biofilm formation in Ureaplasma antibiotic susceptibility and development of bronchopulmonary dysplasia in preterm neonates
- PMID: 23114371
- PMCID: PMC3600059
- DOI: 10.1097/INF.0b013e3182791ae0
Role of biofilm formation in Ureaplasma antibiotic susceptibility and development of bronchopulmonary dysplasia in preterm neonates
Abstract
Background: Ureaplasma respiratory tract colonization is a risk factor for bronchopulmonary dysplasia (BPD) in preterm infants, but whether Ureaplasma isolates from colonized infants can form biofilms is unknown. We hypothesized that Ureaplasma isolates vary in capacity to form biofilms that contribute to their antibiotic resistance and ability to evade host immune responses. Study objectives were to (1) determine the ability of Ureaplasma isolates from preterm neonates to form biofilms in vitro; (2) compare the susceptibility of the sessile and planktonic organisms to azithromycin (AZI) and erythromycin; and (3) determine the relationship of biofilm-forming capacity in Ureaplasma isolates and the risk for BPD.
Methods: Forty-three clinical isolates from preterm neonates and 5 American Tissue Culture Collection strains were characterized for their capacity to form biofilms in vitro, and antibiotic susceptibility was performed on each isolate prebiofilm and postbiofilm formation.
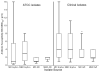
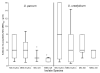
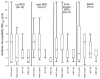
Results: Forty-one (95%) clinical and 4 of 5 (80%) American Tissue Culture Collection isolates formed biofilms. All isolates were more susceptible to AZI (minimum inhibitory concentration, MIC50 2 µg/mL) than erythromycin (MIC50 4 µg/mL), and biofilm formation did not significantly affect antibiotic susceptibility for the 2 tested antibiotics. The MIC50 and minimum biofilm inhibitory concentrations (MBIC50) for Ureaplasma urealyticum clinical isolates for AZI were higher than for MIC50 and MBIC50 for Ureaplasma parvum isolates. There were no differences in MIC or MBICs among isolates from BPD infants and non-BPD infants.
Conclusions: Capacity to form biofilms is common among Ureaplasma spp. isolates, but biofilm formation did not impact MICs for AZI or erythromycin.
Figures
References
-
- Volgmann T, Ohlinger R, Panzig B. Ureaplasma urealyticum-harmless commensal or underestimated enemy of human reproduction? A review. Arch Gynecol Obstet. 2005;273:133–139. - PubMed
-
- Namba F, Hasegawa T, Nakayama M, et al. Placental features of chorioamnionitis colonized with Ureaplasma species in preterm delivery. Pediatr Res. 2010;67:166–172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

