Acceptability of potential rectal microbicide delivery systems for HIV prevention: a randomized crossover trial
- PMID: 23114512
- PMCID: PMC3594349
- DOI: 10.1007/s10461-012-0358-z
Acceptability of potential rectal microbicide delivery systems for HIV prevention: a randomized crossover trial
Abstract
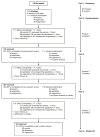
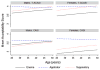
We assessed the acceptability of three of over-the-counter products representative of potential rectal microbicide (RM) delivery systems. From 2009 to 2010, 117 HIV-uninfected males (79 %) and females (21 %) who engage in receptive anal intercourse participated in a 6-week randomized crossover acceptability trial. Participants received each of three products (enema, lubricant-filled applicator, suppository) every 2 weeks in a randomized sequence. CASI and T-ACASI scales assessed product acceptability via Likert responses. Factor analysis was used to identify underlying factors measured by each scale. Random effects models were fit to examine age and gender effects on product acceptability. Three underlying factors were identified: Satisfaction with Product Use, Sexual Pleasure, and Ease of Product Use. For acceptability, the applicator ranked highest; however, differences between product acceptability scores were greatest among females and younger participants. These findings indicate that RM delivery systems impact their acceptability and should be considered early in RM development to enhance potential use.
Figures
Similar articles
-
Rectal-specific microbicide applicator: evaluation and comparison with a vaginal applicator used rectally.AIDS Behav. 2014 Sep;18(9):1734-45. doi: 10.1007/s10461-014-0793-0. AIDS Behav. 2014. PMID: 24858481 Free PMC article.
-
A randomized trial of safety, acceptability and adherence of three rectal microbicide placebo formulations among young sexual and gender minorities who engage in receptive anal intercourse (MTN-035).PLoS One. 2023 Apr 12;18(4):e0284339. doi: 10.1371/journal.pone.0284339. eCollection 2023. PLoS One. 2023. PMID: 37043527 Free PMC article. Clinical Trial.
-
Condoms, Lubricants and Rectal Cleansing: Practices Associated with Heterosexual Penile-Anal Intercourse Amongst Participants in an HIV Prevention Trial in South Africa, Uganda and Zimbabwe.AIDS Behav. 2016 Apr;20(4):754-62. doi: 10.1007/s10461-015-1120-0. AIDS Behav. 2016. PMID: 26126586 Free PMC article.
-
Acceptability research on female-controlled barrier methods to prevent heterosexual transmission of HIV: Where have we been? Where are we going?J Womens Health Gend Based Med. 2001 Mar;10(2):163-73. doi: 10.1089/152460901300039502. J Womens Health Gend Based Med. 2001. PMID: 11268299 Review.
-
Formulation and delivery of anti-HIV rectal microbicides: advances and challenges.J Control Release. 2014 Nov 28;194:278-94. doi: 10.1016/j.jconrel.2014.09.013. Epub 2014 Sep 16. J Control Release. 2014. PMID: 25229988 Review.
Cited by
-
Transcutaneously refillable nanofluidic implant achieves sustained level of tenofovir diphosphate for HIV pre-exposure prophylaxis.J Control Release. 2018 Sep 28;286:315-325. doi: 10.1016/j.jconrel.2018.08.010. Epub 2018 Aug 6. J Control Release. 2018. PMID: 30092254 Free PMC article.
-
Designing Dual Compartment HIV Prevention Products: Women's Sensory Perceptions and Experiences of Suppositories for Rectal and Vaginal Use.AIDS Res Hum Retroviruses. 2022 Jul;38(7):601-610. doi: 10.1089/AID.2021.0038. Epub 2021 Oct 18. AIDS Res Hum Retroviruses. 2022. PMID: 34544269 Free PMC article.
-
Overcoming COVID-19 disruptions: Innovations in product provision in a multi-national clinical trial among cisgender men, transgender men and transgender women in five countries.Contemp Clin Trials Commun. 2022 Aug;28:100930. doi: 10.1016/j.conctc.2022.100930. Epub 2022 Jun 6. Contemp Clin Trials Commun. 2022. PMID: 35693378 Free PMC article.
-
History of Rectal Product Use and Country of Residence Influence Preference for Rectal Microbicide Dosage Forms Among Young Sexual and Gender Minorities: A Multi-country Trial Comparing Placebo Douche, Suppository, and Insert Products.AIDS Behav. 2024 Aug;28(8):2577-2589. doi: 10.1007/s10461-024-04360-9. Epub 2024 May 13. AIDS Behav. 2024. PMID: 38740628 Free PMC article. Clinical Trial.
-
Rectal pre-exposure prophylaxis (PrEP).Antiviral Res. 2013 Dec;100 Suppl(0):S17-24. doi: 10.1016/j.antiviral.2013.09.023. Epub 2013 Nov 1. Antiviral Res. 2013. PMID: 24188705 Free PMC article. Review.
References
-
- Rosenberger JG, Reece M, Schick V, et al. Sexual behaviors and situational characteristics of most recent male-partnered sexual event among gay and bisexually identified men in the United States. J Sex Med. 2011;8(11):3040–50. - PubMed
-
- Reece M, Herbenick D, Schick V, Sanders SA, Dodge B, Fortenberry JD. Condom use rates in a national probability sample of males and females ages 14 to 94 in the United States. J Sex Med. 2010;7(Suppl 5):266–76. - PubMed
-
- Herbenick D, Reece M, Schick V, Sanders SA, Dodge B, Fortenberry JD. Sexual behavior in the United States: results from a national probability sample of men and women ages 14–94. J Sex Med. 2010;7(Suppl 5):255–65. - PubMed
-
- Vittinghoff E, Douglas J, Judson F, McKirnan D, MacQueen K, Buchbinder SP. Per-contact risk of human immunodeficiency virus transmission between male sexual partners. Am J Epidemiol. 1999;150(3):306–11. - PubMed
-
- Varghese B, Maher JE, Peterman TA, Branson BM, Steketee RW. Reducing the risk of sexual HIV transmission: quantifying the per-act risk for HIV on the basis of choice of partner, sex act, and condom use. Sex Transm Dis. 2002;29(1):38–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

