Natriuretic peptides enhance the oxidative capacity of human skeletal muscle
- PMID: 23114600
- PMCID: PMC3533552
- DOI: 10.1172/JCI64526
Natriuretic peptides enhance the oxidative capacity of human skeletal muscle
Abstract
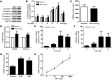
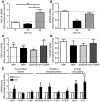
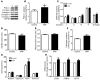
Cardiac natriuretic peptides (NP) are major activators of human fat cell lipolysis and have recently been shown to control brown fat thermogenesis. Here, we investigated the physiological role of NP on the oxidative metabolism of human skeletal muscle. NP receptor type A (NPRA) gene expression was positively correlated to mRNA levels of PPARγ coactivator-1α (PGC1A) and several oxidative phosphorylation (OXPHOS) genes in human skeletal muscle. Further, the expression of NPRA, PGC1A, and OXPHOS genes was coordinately upregulated in response to aerobic exercise training in human skeletal muscle. In human myotubes, NP induced PGC-1α and mitochondrial OXPHOS gene expression in a cyclic GMP-dependent manner. NP treatment increased OXPHOS protein expression, fat oxidation, and maximal respiration independent of substantial changes in mitochondrial proliferation and mass. Treatment of myotubes with NP recapitulated the effect of exercise training on muscle fat oxidative capacity in vivo. Collectively, these data show that activation of NP signaling in human skeletal muscle enhances mitochondrial oxidative metabolism and fat oxidation. We propose that NP could contribute to exercise training-induced improvement in skeletal muscle fat oxidative capacity in humans.
Figures
References
-
- Gardner DG. Natriuretic peptides: markers or modulators of cardiac hypertrophy? Trends Endocrinol Metab. 2003;14(9):411–416. - PubMed
-
- Lafontan M, Moro C, Berlan M, Crampes F, Sengenes C, Galitzky J. Control of lipolysis by natriuretic peptides and cyclic GMP. Trends Endocrinol Metab. 2008;19(4):130–137. - PubMed
-
- Sengenes C, et al. Natriuretic peptide-dependent lipolysis in fat cells is a primate specificity. Am J Physiol Regul Integr Comp Physiol. 2002;283(1):R257–R265. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

