Advances in stem cell therapy for spinal cord injury
- PMID: 23114605
- PMCID: PMC3484454
- DOI: 10.1172/JCI64124
Advances in stem cell therapy for spinal cord injury
Abstract
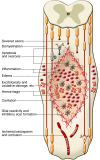
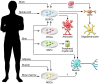
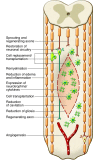
Spinal cord injury (SCI) is a devastating condition producing great personal and societal costs and for which there is no effective treatment. Stem cell transplantation is a promising therapeutic strategy, though much preclinical and clinical research work remains. Here, we briefly describe SCI epidemiology, pathophysiology, and experimental and clinical stem cell strategies. Research in stem cell biology and cell reprogramming is rapidly advancing, with the hope of moving stem cell therapy closer to helping people with SCI. We examine issues important for clinical translation and provide a commentary on recent developments, including termination of the first human embryonic stem cell transplantation trial in human SCI.
Figures
References
-
- Tator CH. Review of treatment trials in human spinal cord injury: issues, difficulties, and recommendations. Neurosurgery. 2006;59(5):957–982. - PubMed

