Benznidazole-related adverse drug reactions and their relationship to serum drug concentrations in patients with chronic chagas disease
- PMID: 23114763
- PMCID: PMC3535922
- DOI: 10.1128/AAC.01401-12
Benznidazole-related adverse drug reactions and their relationship to serum drug concentrations in patients with chronic chagas disease
Abstract
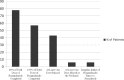
For treating Chagas disease (CD), a current worldwide health problem, only benznidazole and nifurtimox have been approved to be used. In both cases, unwanted drug-related adverse events (ADRs) are frequent when these drugs are used in adults in the chronic stage. The main objective of this study was to establish benznidazole ADRs and their relationship to serum concentrations in patients with chronic Trypanosoma cruzi infection in order to perform more accurate dosages to minimize ADRs. A total of 54 patients were recruited over 12 months. Of these 54 patients, 53 (98%) experienced at least one ADR during follow-up, and the overall average ADR incidence was 2.4 episodes/patient/month. Benznidazole treatment was discontinued in 11 patients, 7 among them due to severe adverse effects. The mean duration of treatment before withdrawal was 11 days. Benznidazole serum concentrations were recorded on days 15, 30, 45, and 60 of follow-up and evaluated according to clinical and epidemiological variables and ADR severity. No relationship was found between the benznidazole serum concentration and the ADRs. The mean (standard deviation) trough serum benznidazole concentrations (all below 20 mcg/ml) on days 15, 30, 45, and 60 were 6.4 (1.9), 6.1 (1.8), 6.2 (2.2), and 5.7 (1.7) μg/ml, respectively. Benznidazole serum concentrations do not appear to be related to the appearance of serious ADRs. Further, well-controlled studies are necessary to establish the optimal regimen for benznidazole in adults with chronic CD.
Figures
References
-
- Pan American Health Organization 2006. Estimación cuantitativa de la enfermedad de Chagas en las Americas. Organización Panamericana de la Salud, Montevideo, Uruguay
-
- Gascon J, Bern C, Pinazo MJ. 2010. Chagas disease in Spain, the United States, and other non-endemic countries. Acta Trop. 115:22–27 - PubMed
-
- Prata A. 2001. Clinical and epidemiological aspects of Chagas disease. Lancet Infect. Dis. 1:92–100 - PubMed
-
- Schijman AG, Altcheh J, Burgos JM, Biancardi M, Bisio M, Levin MJ, Freilij H. 2003. Aetiological treatment of congenital Chagas' disease diagnosed and monitored by the polymerase chain reaction. J. Antimicrob. Chemother. 52:441–449 - PubMed
-
- de Andrade AL, Zicker de Oliveira FRM, Almeida Silva S, Luquetti A, Travassos LR, Almeida IC, de Andrade SS, de Andrade JG, Martelli CM. 1996. Randomised trial of efficacy of benznidazole in treatment of early Trypanosoma cruzi infection. Lancet 348:1407–1413 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials