Heading off with the herd: how cancer cells might maneuver supernumerary centrosomes for directional migration
- PMID: 23114845
- PMCID: PMC3581755
- DOI: 10.1007/s10555-012-9413-5
Heading off with the herd: how cancer cells might maneuver supernumerary centrosomes for directional migration
Abstract
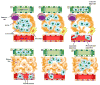
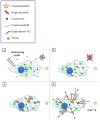
The complicity of centrosomes in carcinogenesis is unmistakable. Mounting evidence clearly implicates a robust correlation between centrosome amplification (CA) and malignant transformation in diverse tissue types. Furthermore, CA has been suggested as a marker of cancer aggressiveness, in particular the invasive phenotype, in breast and prostate cancers. One means by which CA promotes malignancy is through induction of transient spindle multipolarity during mitosis, which predisposes the cell to karyotypic changes arising from low-grade chromosome mis-segregation. It is well recognized that during cell migration in interphase, centrosome-mediated nucleation of a radial microtubule array is crucial for establishing a polarized Golgi apparatus, without which directionality is precluded. The question of how cancer cells maneuver their supernumerary centrosomes to achieve directionality during cell migration is virtually uncharted territory. Given that CA is a hallmark of cancers and has been correlated with cancer aggressiveness, malignant cells are presumably competent in managing their centrosome surfeit during directional migration, although the cellular logistics of this process remain unexplored. Another key angle worth pondering is whether an overabundance of centrosomes confers some advantage on cancer cells in terms of their migratory and invasive capabilities. Recent studies have uncovered a remarkable strategy that cancer cells employ to deal with the problem of excess centrosomes and ensure bipolar mitoses, viz., centrosome clustering. This review aims to change the narrative by exploring how an increased centrosome complement may, via aneuploidy-independent modulation of the microtubule cytoskeleton, enhance directional migration and invasion of malignant cells. We postulate that CA imbues cancer cells with cytoskeletal advantages that enhance cell polarization, Golgi-dependent vesicular trafficking, stromal invasion, and other aspects of metastatic progression. We also propose that centrosome declustering may represent a novel, cancer cell-specific antimetastatic strategy, as cancer cells may rely on centrosome clustering during migration as they do in mitosis. Elucidation of these details offers an exciting avenue for future research, as does investigating how CA may promote metastasis through enhanced directional migration.
Figures


References
-
- D’Assoro AB, Lingle WL, Salisbury JL. Centrosome amplification and the development of cancer. Oncogene. 2002;21(40):6146–53. - PubMed
-
- Prigozhina NL, Waterman-Storer CM. Decreased polarity and increased random motility in PtK1 epithelial cells correlate with inhibition of endosomal recycling. J Cell Sci. 2006;119(Pt 17):3571–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

