Expression of hepatocyte epidermal growth factor receptor, FAS and glypican 3 in EpCAM-positive regenerative clusters of hepatocytes, cholangiocytes, and progenitor cells in human liver failure
- PMID: 23114924
- PMCID: PMC3635113
- DOI: 10.1016/j.humpath.2012.07.018
Expression of hepatocyte epidermal growth factor receptor, FAS and glypican 3 in EpCAM-positive regenerative clusters of hepatocytes, cholangiocytes, and progenitor cells in human liver failure
Abstract
Liver regeneration under normal circumstances proceeds through proliferation of all cellular elements of the liver. Studies with rodent models have shown that when proliferation of hepatocytes is inhibited, progenitor cells arising from the biliary compartment transdifferentiate into "oval/progenitor" cells, which proceed to differentiate into hepatocytes. Recent studies have shown that the same pathways may operate in human liver failure. The growth factor receptors (HGF [hepatocyte growth factor] receptor) and epidermal growth factor receptor are key mitogenic receptors for both hepatocytes and progenitor cells. Our current study used the biliary and progenitor marker EpCAM (epithelial cell adhesion molecule) to detect "regenerative clusters" of mixed cholangiocyte-hepatocyte differentiation. We determined that expression of metabolic equivalent and epidermal growth factor receptor occurs in biliary cells, progenitor cells, and hepatocytes, whereas activation of metabolic equivalent and epidermal growth factor receptor is limited to regenerative cluster hepatocytes. These histologic events are associated with expression of apoptosis-inducing FAS and mitoinhibitory protein glypican 3. Cell proliferation was overall suppressed in regenerative clusters. Transdifferentiation of biliary and progenitor cells appears to be regulated by a complex interaction of signals promoting and arresting growth.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
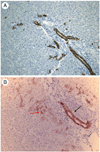

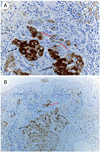
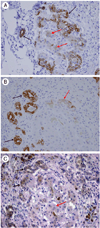
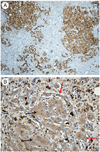


References
-
- Evarts RP, Nagy P, Nakatsukasa H, Marsden E, Thorgeirsson SS. In vivo differentiation of rat liver oval cells into hepatocytes. Cancer Res. 1989;49:1541–1547. - PubMed
-
- Golding M, Sarraf CE, Lalani EN, et al. Oval cell differentiation into hepatocytes in the acetylaminofluorene-treated regenerating rat liver. Hepatology. 1995;22:1243–1253. - PubMed
-
- Bisgaard HC, Nagy P, Santoni-Rugiu E, Thorgeirsson SS. Proliferation, apoptosis, and induction of hepatic transcription factors are characteristics of the early response of biliary epithelial (oval) cells to chemical carcinogens. Hepatology. 1996;23:62–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

