O-Mannosylation and human disease
- PMID: 23115008
- PMCID: PMC3984002
- DOI: 10.1007/s00018-012-1193-0
O-Mannosylation and human disease
Abstract
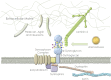
Glycosylation of proteins is arguably the most prevalent co- and post-translational modification. It is responsible for increased heterogeneity and functional diversity of proteins. Here we discuss the importance of one type of glycosylation, specifically O-mannosylation and its relationship to a number of human diseases. The most widely studied O-mannose modified protein is alpha-dystroglycan (α-DG). Recent studies have focused intensely on α-DG due to the severity of diseases associated with its improper glycosylation. O-mannosylation of α-DG is involved in cancer metastasis, arenavirus entry, and multiple forms of congenital muscular dystrophy [1, 2]. In this review, we discuss the structural and functional characteristics of O-mannose-initiated glycan structures on α-DG, enzymes involved in the O-mannosylation pathway, and the diseases that are a direct result of disruptions within this pathway.
Figures

References
-
- Hara Y, Balci-Hayta B, Yoshida-Moriguchi T, Kanagawa M, Beltrán-Valero de Bernabé D, Gündeşli H, Willer T, Satz JS, Crawford RW, Burden SJ, Kunz S, Oldstone MB, Accardi A, Talim B, Muntoni F, Topaloğlu H, Dinçer P, Campbell KP. A dystroglycan mutation associated with limb-girdle muscular dystrophy. N Engl J Med. 2011;364:939–946. doi: 10.1056/NEJMoa1006939. - DOI - PMC - PubMed
-
- Finne J, Krusius T, Margolis RK, Margolis RU. Novel mannitol-containing oligosaccharides obtained by mild alkaline borohydride treatment of a chondroitin sulfate proteoglycan from brain. J Biol Chem. 1979;254:10295–10300. - PubMed
-
- Chiba A, Matsumura K, Yamada H, Inazu T, Shimizu T, Kusunoki S, Kanazawa I, Kobata A, Endo T. Structures of sialylated O-linked oligosaccharides of bovine peripheral nerve alpha-dystroglycan. The role of a novel O-mannosyl-type oligosaccharide in the binding of alpha-dystroglycan with laminin. J Biol Chem. 1997;272:2156–2162. doi: 10.1074/jbc.272.4.2156. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

