Enterococcus faecalis inhibits hyphal morphogenesis and virulence of Candida albicans
- PMID: 23115035
- PMCID: PMC3536143
- DOI: 10.1128/IAI.00914-12
Enterococcus faecalis inhibits hyphal morphogenesis and virulence of Candida albicans
Abstract
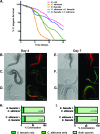
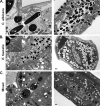
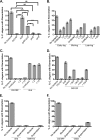
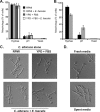
The Gram-positive bacterium Enterococcus faecalis and the fungus Candida albicans are both found as commensals in many of the same niches of the human body, such as the oral cavity and gastrointestinal (GI) tract. However, both are opportunistic pathogens and have frequently been found to be coconstituents of polymicrobial infections. Despite these features in common, there has been little investigation into whether these microbes affect one another in a biologically significant manner. Using a Caenorhabditis elegans model of polymicrobial infection, we discovered that E. faecalis and C. albicans negatively impact each other's virulence. Much of the negative effect of E. faecalis on C. albicans was due to the inhibition of C. albicans hyphal morphogenesis, a developmental program crucial to C. albicans pathogenicity. We discovered that the inhibition was partially dependent on the Fsr quorum-sensing system, a major regulator of virulence in E. faecalis. Specifically, two proteases regulated by Fsr, GelE and SerE, were partially required. Further characterization of the inhibitory signal revealed that it is secreted into the supernatant, is heat resistant, and is between 3 and 10 kDa. The substance was also shown to inhibit C. albicans filamentation in the context of an in vitro biofilm. Finally, a screen of an E. faecalis transposon mutant library identified other genes required for suppression of C. albicans hyphal formation. Overall, we demonstrate a biologically relevant interaction between two clinically important microbes that could affect treatment strategies as well as impact our understanding of interkingdom signaling and sensing in the human-associated microbiome.
Figures


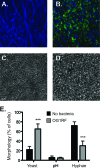
Comment in
-
Candida albicans and Enterococcus faecalis in the gut: synergy in commensalism?Gut Microbes. 2013 Sep-Oct;4(5):409-15. doi: 10.4161/gmic.26040. Epub 2013 Aug 14. Gut Microbes. 2013. PMID: 23941906 Free PMC article.
References
-
- Morales DK, Hogan DA. 2010. Candida albicans interactions with bacteria in the context of human health and disease. PLoS Pathog. 6:e1000886 doi:10.1371/journal.ppat.1000886 - DOI - PMC - PubMed
-
- Peleg AY, Hogan DA, Mylonakis E. 2010. Medically important bacterial-fungal interactions. Nat. Rev. Microbiol. 8:340–349 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

