Detection of glioblastoma response to temozolomide combined with bevacizumab based on μMRI and μPET imaging reveals [18F]-fluoro-L-thymidine as an early and robust predictive marker for treatment efficacy
- PMID: 23115160
- PMCID: PMC3534417
- DOI: 10.1093/neuonc/nos260
Detection of glioblastoma response to temozolomide combined with bevacizumab based on μMRI and μPET imaging reveals [18F]-fluoro-L-thymidine as an early and robust predictive marker for treatment efficacy
Abstract
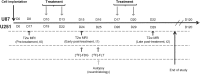
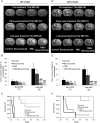
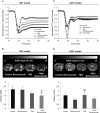
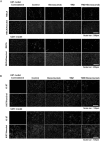
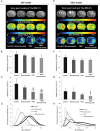
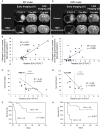
The individualized care of glioma patients ought to benefit from imaging biomarkers as precocious predictors of therapeutic efficacy. Contrast enhanced MRI and [(18)F]-fluorodeoxyglucose (FDG)-PET are routinely used in clinical settings; their ability to forecast the therapeutic response is controversial. The objectives of our preclinical study were to analyze sensitive µMRI and/or µPET imaging biomarkers to predict the efficacy of anti-angiogenic and/or chemotherapeutic regimens. Human U87 and U251 orthotopic glioma models were implanted in nude rats. Temozolomide and/or bevacizumab were administered. µMRI (anatomical, diffusion, and microrheological parameters) and µPET ([(18)F]-FDG and [(18)F]-fluoro-l-thymidine [FLT]-PET) studies were undertaken soon (t(1)) after treatment initiation compared with late anatomical µMRI evaluation of tumor volume (t(2)) and overall survival. In both models, FDG and FLT uptakes were attenuated at t(1) in response to temozolomide alone or with bevacizumab. The distribution of FLT, reflecting intratumoral heterogeneity, was also modified. FDG was less predictive for treatment efficacy than was FLT (also highly correlated with outcome, P < .001 for both models). Cerebral blood volume was significantly decreased by temozolomide + bevacizumab and was correlated with survival for rats with U87 implants. While FLT was highly predictive of treatment efficacy, a combination of imaging biomarkers was superior to any one alone (P < .0001 in both tumors with outcome). Our results indicate that FLT is a sensitive predictor of treatment efficacy and that predictability is enhanced by a combination of imaging biomarkers. These findings may translate clinically in that individualized glioma treatments could be decided in given patients after PET/MRI examinations.
Figures

References
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Desjardins A, Friedman HS. Neuro-oncology: glioblastoma-community adjusts to new standard of care. Nat Rev Neurol. 2012;8:244–246. - PubMed
-
- Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8:610–622. - PubMed
-
- Miletic H, Niclou SP, Johansson M, Bjerkvig R. Anti-VEGF therapies for malignant glioma: treatment effects and escape mechanisms. Expert Opin Ther Targets. 2009;13:455–468. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

