Regulation of neuronal excitability by interaction of fragile X mental retardation protein with slack potassium channels
- PMID: 23115170
- PMCID: PMC3518385
- DOI: 10.1523/JNEUROSCI.2162-12.2012
Regulation of neuronal excitability by interaction of fragile X mental retardation protein with slack potassium channels
Abstract
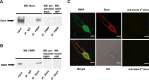
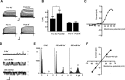
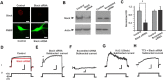
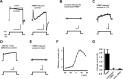
Loss of the RNA-binding protein fragile X mental retardation protein (FMRP) represents the most common form of inherited intellectual disability. Studies with heterologous expression systems indicate that FMRP interacts directly with Slack Na(+)-activated K(+) channels (K(Na)), producing an enhancement of channel activity. We have now used Aplysia bag cell (BC) neurons, which regulate reproductive behaviors, to examine the effects of Slack and FMRP on excitability. FMRP and Slack immunoreactivity were colocalized at the periphery of isolated BC neurons, and the two proteins could be reciprocally coimmunoprecipitated. Intracellular injection of FMRP lacking its mRNA binding domain rapidly induced a biphasic outward current, with an early transient tetrodotoxin-sensitive component followed by a slowly activating sustained component. The properties of this current matched that of the native Slack potassium current, which was identified using an siRNA approach. Addition of FMRP to inside-out patches containing native Aplysia Slack channels increased channel opening and, in current-clamp recordings, produced narrowing of action potentials. Suppression of Slack expression did not alter the ability of BC neurons to undergo a characteristic prolonged discharge in response to synaptic stimulation, but prevented recovery from a prolonged inhibitory period that normally follows the discharge. Recovery from the inhibited period was also inhibited by the protein synthesis inhibitor anisomycin. Our studies indicate that, in BC neurons, Slack channels are required for prolonged changes in neuronal excitability that require new protein synthesis, and raise the possibility that channel-FMRP interactions may link changes in neuronal firing to changes in protein translation.
Figures


Similar articles
-
Fragile X mental retardation protein modulates somatic D-type K+ channels and action potential threshold in the mouse prefrontal cortex.J Neurophysiol. 2020 Dec 1;124(6):1766-1773. doi: 10.1152/jn.00494.2020. Epub 2020 Sep 30. J Neurophysiol. 2020. PMID: 32997566 Free PMC article.
-
An ALS-Associated Mutant SOD1 Rapidly Suppresses KCNT1 (Slack) Na+-Activated K+ Channels in Aplysia Neurons.J Neurosci. 2017 Feb 22;37(8):2258-2265. doi: 10.1523/JNEUROSCI.3102-16.2017. Epub 2017 Jan 24. J Neurosci. 2017. PMID: 28119399 Free PMC article.
-
Loss of Sodium-Activated Potassium Channel Slack and FMRP Differentially Affect Social Behavior in Mice.Neuroscience. 2018 Aug 1;384:361-374. doi: 10.1016/j.neuroscience.2018.05.040. Epub 2018 May 31. Neuroscience. 2018. PMID: 29859980
-
Fragile X mental retardation protein controls ion channel expression and activity.J Physiol. 2016 Oct 15;594(20):5861-5867. doi: 10.1113/JP270675. Epub 2016 May 5. J Physiol. 2016. PMID: 26864773 Free PMC article. Review.
-
Emerging role of the KCNT1 Slack channel in intellectual disability.Front Cell Neurosci. 2014 Jul 28;8:209. doi: 10.3389/fncel.2014.00209. eCollection 2014. Front Cell Neurosci. 2014. PMID: 25120433 Free PMC article. Review.
Cited by
-
Fragile X mental retardation protein modulates somatic D-type K+ channels and action potential threshold in the mouse prefrontal cortex.J Neurophysiol. 2020 Dec 1;124(6):1766-1773. doi: 10.1152/jn.00494.2020. Epub 2020 Sep 30. J Neurophysiol. 2020. PMID: 32997566 Free PMC article.
-
Interactome of FMRP-N-tat therapeutic unveils key interactions for cellular function in Fragile X neurons.J Biol Chem. 2025 Jul;301(7):110341. doi: 10.1016/j.jbc.2025.110341. Epub 2025 Jun 4. J Biol Chem. 2025. PMID: 40480633 Free PMC article.
-
A sodium-activated potassium channel supports high-frequency firing and reduces energetic costs during rapid modulations of action potential amplitude.J Neurophysiol. 2013 Apr;109(7):1713-23. doi: 10.1152/jn.00875.2012. Epub 2013 Jan 16. J Neurophysiol. 2013. PMID: 23324315 Free PMC article.
-
The sodium-activated potassium channel Slack is required for optimal cognitive flexibility in mice.Learn Mem. 2015 Jun 15;22(7):323-35. doi: 10.1101/lm.037820.114. Print 2015 Jul. Learn Mem. 2015. PMID: 26077685 Free PMC article.
-
Characterization of Fragile X Mental Retardation Protein expression in human nociceptors and their axonal projections to the spinal dorsal horn.J Comp Neurol. 2023 May;531(7):814-835. doi: 10.1002/cne.25463. Epub 2023 Feb 20. J Comp Neurol. 2023. PMID: 36808110 Free PMC article.
References
-
- An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, Hinson JW, Mattsson KI, Strassle BW, Trimmer JS, Rhodes KJ. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–556. - PubMed
-
- Bader CR, Bernheim L, Bertrand D. Sodium-activated potassium current in cultured avian neurones. Nature. 1985;317:540–542. - PubMed
-
- Bagni C, Greenough WT. From mRNP trafficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nat Rev Neurosci. 2005;6:376–387. - PubMed
-
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
