Mammalian target of rapamycin (mTOR) signaling network in skeletal myogenesis
- PMID: 23115234
- PMCID: PMC3527976
- DOI: 10.1074/jbc.R112.406942
Mammalian target of rapamycin (mTOR) signaling network in skeletal myogenesis
Abstract
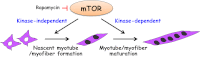
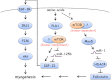
Mammalian (or mechanistic) target of rapamycin (mTOR) regulates a wide range of cellular and developmental processes by coordinating signaling responses to mitogens, nutrients, and various stresses. Over the last decade, mTOR has emerged as a master regulator of skeletal myogenesis, controlling multiple stages of the myofiber formation process. In this minireview, we present an emerging view of the signaling network underlying mTOR regulation of myogenesis, which contrasts with the well established mechanisms in the regulation of cell and muscle growth. Current questions for future studies are also highlighted.
Figures

References
-
- Martin D. E., Hall M. N. (2005) The expanding TOR signaling network. Curr. Opin. Cell Biol. 17, 158–166 - PubMed
-
- Sarbassov D. D., Ali S. M., Sabatini D. M. (2005) Growing roles for the mTOR pathway. Curr. Opin. Cell Biol. 17, 596–603 - PubMed
-
- Ma X. M., Blenis J. (2009) Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 10, 307–318 - PubMed
-
- Hsu P. P., Kang S. A., Rameseder J., Zhang Y., Ottina K. A., Lim D., Peterson T. R., Choi Y., Gray N. S., Yaffe M. B., Marto J. A., Sabatini D. M. (2011) The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science 332, 1317–1322 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

