The mRNA-stabilizing factor HuR protein is targeted by β-TrCP protein for degradation in response to glycolysis inhibition
- PMID: 23115237
- PMCID: PMC3527950
- DOI: 10.1074/jbc.M112.393678
The mRNA-stabilizing factor HuR protein is targeted by β-TrCP protein for degradation in response to glycolysis inhibition
Abstract
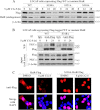
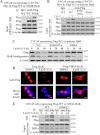
The mRNA-stabilizing protein HuR acts a stress response protein whose function and/or protein stability are modulated by diverse stress stimuli through posttranslational modifications. Here, we report a novel mechanism by which metabolic stress facilitates proteasomal degradation of HuR in cancer cells. In response to the glucose transporter inhibitor CG-5, HuR translocates to the cytoplasm, where it is targeted by the ubiquitin E3 ligase β-TrCP1 for degradation. The cytoplasmic localization of HuR is facilitated by PKCα-mediated phosphorylation at Ser-318 as the Ser-318 → alanine substitution abolishes the ability of the resulting HuR to bind PKCα and to undergo nuclear export. The mechanistic link between β-TrCP1 and HuR degradation was supported by the ability of ectopically expressed β-TrCP1 to mimic CG-5 to promote HuR degradation and by the protective effect of dominant negative inhibition of β-TrCP1 on HuR ubiquitination and degradation. Substrate targeting of HuR by β-TrCP1 was further verified by coimmunoprecipitation and in vitro GST pull-down assays and by the identification of a β-TrCP1 recognition site. Although HuR does not contain a DSG destruction motif, we obtained evidence that β-TrCP1 recognizes an unconventional motif, (296)EEAMAIAS(304), in the RNA recognition motif 3. Furthermore, mutational analysis indicates that IKKα-dependent phosphorylation at Ser-304 is crucial to the binding of HuR to β-TrCP1. Mechanistically, this HuR degradation pathway differs from that reported for heat shock and hypoxia, which underlies the complexity in the regulation of HuR turnover under different stress stimuli. The ability of glycolysis inhibitors to target the expression of oncogenic proteins through HuR degradation might foster novel strategies for cancer therapy.
Figures




Similar articles
-
A novel mechanism by which thiazolidinediones facilitate the proteasomal degradation of cyclin D1 in cancer cells.J Biol Chem. 2008 Sep 26;283(39):26759-70. doi: 10.1074/jbc.M802160200. Epub 2008 Jul 23. J Biol Chem. 2008. PMID: 18650423 Free PMC article.
-
The cross talk of two family members of β-TrCP in the regulation of cell autophagy and growth.Cell Death Differ. 2020 Mar;27(3):1119-1133. doi: 10.1038/s41418-019-0402-x. Epub 2019 Aug 13. Cell Death Differ. 2020. PMID: 31406304 Free PMC article.
-
Mislocalization of the E3 ligase, β-transducin repeat-containing protein 1 (β-TrCP1), in glioblastoma uncouples negative feedback between the pleckstrin homology domain leucine-rich repeat protein phosphatase 1 (PHLPP1) and Akt.J Biol Chem. 2011 Jun 3;286(22):19777-88. doi: 10.1074/jbc.M111.237081. Epub 2011 Mar 28. J Biol Chem. 2011. PMID: 21454620 Free PMC article.
-
The characteristics and roles of β-TrCP1/2 in carcinogenesis.FEBS J. 2021 Jun;288(11):3351-3374. doi: 10.1111/febs.15585. Epub 2020 Oct 23. FEBS J. 2021. PMID: 33021036 Review.
-
Regulation of NF-κB by ubiquitination and degradation of the IκBs.Immunol Rev. 2012 Mar;246(1):77-94. doi: 10.1111/j.1600-065X.2012.01098.x. Immunol Rev. 2012. PMID: 22435548 Review.
Cited by
-
Insulin-like growth factor-I receptor is suppressed through transcriptional repression and mRNA destabilization by a novel energy restriction-mimetic agent.Carcinogenesis. 2013 Dec;34(12):2694-705. doi: 10.1093/carcin/bgt251. Epub 2013 Jul 16. Carcinogenesis. 2013. Retraction in: Carcinogenesis. 2019 Apr 29;40(2):e14. doi: 10.1093/carcin/bgz055. PMID: 23864387 Free PMC article. Retracted.
-
α4 Coordinates Small Intestinal Epithelium Homeostasis by Regulating Stability of HuR.Mol Cell Biol. 2018 May 15;38(11):e00631-17. doi: 10.1128/MCB.00631-17. Print 2018 Jun 1. Mol Cell Biol. 2018. PMID: 29555726 Free PMC article.
-
Posttranslational control of HuR function.Wiley Interdiscip Rev RNA. 2017 Jan;8(1):10.1002/wrna.1372. doi: 10.1002/wrna.1372. Epub 2016 Jun 16. Wiley Interdiscip Rev RNA. 2017. PMID: 27307117 Free PMC article. Review.
-
Role and prognostic significance of the epithelial-mesenchymal transition factor ZEB2 in ovarian cancer.Oncotarget. 2015 Aug 7;6(22):18966-79. doi: 10.18632/oncotarget.3943. Oncotarget. 2015. PMID: 26136338 Free PMC article.
-
Understanding and targeting the disease-related RNA binding protein human antigen R (HuR).Wiley Interdiscip Rev RNA. 2020 May;11(3):e1581. doi: 10.1002/wrna.1581. Epub 2020 Jan 23. Wiley Interdiscip Rev RNA. 2020. PMID: 31970930 Free PMC article. Review.
References
-
- Song I. S., Tatebe S., Dai W., Kuo M. T. (2005) Delayed mechanism for induction of γ-glutamylcysteine synthetase heavy subunit mRNA stability by oxidative stress involving p38 mitogen-activated protein kinase signaling. J. Biol. Chem. 280, 28230–28240 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

