Gain-of-function Nav1.8 mutations in painful neuropathy
- PMID: 23115331
- PMCID: PMC3511073
- DOI: 10.1073/pnas.1216080109
Gain-of-function Nav1.8 mutations in painful neuropathy
Abstract
Painful peripheral neuropathy often occurs without apparent underlying cause. Gain-of-function variants of sodium channel Na(v)1.7 have recently been found in ∼30% of cases of idiopathic painful small-fiber neuropathy. Here, we describe mutations in Na(v)1.8, another sodium channel that is specifically expressed in dorsal root ganglion (DRG) neurons and peripheral nerve axons, in patients with painful neuropathy. Seven Na(v)1.8 mutations were identified in 9 subjects within a series of 104 patients with painful predominantly small-fiber neuropathy. Three mutations met criteria for potential pathogenicity based on predictive algorithms and were assessed by voltage and current clamp. Functional profiling showed that two of these three Na(v)1.8 mutations enhance the channel's response to depolarization and produce hyperexcitability in DRG neurons. These observations suggest that mutations of Na(v)1.8 contribute to painful peripheral neuropathy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
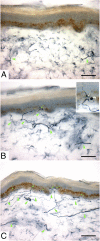

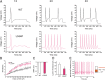

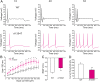
References
-
- Hoeijmakers JG, Faber CG, Lauria G, Merkies IS, Waxman SG. Small-fibre neuropathies—advances in diagnosis, pathophysiology and management. Nat Rev Neurol. 2012;8(7):369–379. - PubMed
-
- Faber CG, et al. Gain of function Naν1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol. 2012;71(1):26–39. - PubMed
-
- Lauria G, et al. Intraepidermal nerve fiber density at the distal leg: A worldwide normative reference study. J Peripher Nerv Syst. 2010;15(3):202–207. - PubMed
-
- Reulen JP, Lansbergen MD, Verstraete E, Spaans F. Comparison of thermal threshold tests to assess small nerve fiber function: Limits vs. levels. Clin Neurophysiol. 2003;114(3):556–563. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

