Cartilage tissue engineering using differentiated and purified induced pluripotent stem cells
- PMID: 23115336
- PMCID: PMC3511083
- DOI: 10.1073/pnas.1210422109
Cartilage tissue engineering using differentiated and purified induced pluripotent stem cells
Abstract
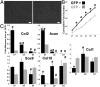
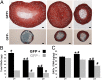
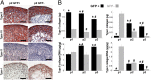
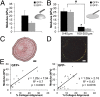
The development of regenerative therapies for cartilage injury has been greatly aided by recent advances in stem cell biology. Induced pluripotent stem cells (iPSCs) have the potential to provide an abundant cell source for tissue engineering, as well as generating patient-matched in vitro models to study genetic and environmental factors in cartilage repair and osteoarthritis. However, both cell therapy and modeling approaches require a purified and uniformly differentiated cell population to predictably recapitulate the physiological characteristics of cartilage. Here, iPSCs derived from adult mouse fibroblasts were chondrogenically differentiated and purified by type II collagen (Col2)-driven green fluorescent protein (GFP) expression. Col2 and aggrecan gene expression levels were significantly up-regulated in GFP+ cells compared with GFP- cells and decreased with monolayer expansion. An in vitro cartilage defect model was used to demonstrate integrative repair by GFP+ cells seeded in agarose, supporting their potential use in cartilage therapies. In chondrogenic pellet culture, cells synthesized cartilage-specific matrix as indicated by high levels of glycosaminoglycans and type II collagen and low levels of type I and type X collagen. The feasibility of cell expansion after initial differentiation was illustrated by homogenous matrix deposition in pellets from twice-passaged GFP+ cells. Finally, atomic force microscopy analysis showed increased microscale elastic moduli associated with collagen alignment at the periphery of pellets, mimicking zonal variation in native cartilage. This study demonstrates the potential use of iPSCs for cartilage defect repair and for creating tissue models of cartilage that can be matched to specific genetic backgrounds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Hunziker EB. Articular cartilage repair: Basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10(6):432–463. - PubMed
-
- Guilak F, Butler DL, Goldstein SA. Functional tissue engineering: the role of biomechanics in articular cartilage repair. Clin Orthop Relat Res. 2001;(391)(Suppl):S295–S305. - PubMed
-
- Song L, Baksh D, Tuan RS. Mesenchymal stem cell-based cartilage tissue engineering: cells, scaffold and biology. Cytotherapy. 2004;6(6):596–601. - PubMed
-
- Brittberg M, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–895. - PubMed
-
- Lee CR, Grodzinsky AJ, Hsu HP, Martin SD, Spector M. Effects of harvest and selected cartilage repair procedures on the physical and biochemical properties of articular cartilage in the canine knee. J Orthop Res. 2000;18(5):790–799. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

