Efficacy of eight months of nightly zolpidem: a prospective placebo-controlled study
- PMID: 23115404
- PMCID: PMC3466802
- DOI: 10.5665/sleep.2208
Efficacy of eight months of nightly zolpidem: a prospective placebo-controlled study
Abstract
Study objectives: To evaluate the long-term (8 months) efficacy of zolpidem in adults with chronic primary insomnia using polysomnography.
Design: Randomized, double-blind, placebo-controlled clinical trial.
Setting: Sleep disorders and research center.
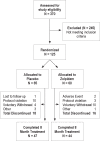
Participants: Healthy participants (n = 91), ages 23-70, meeting DSM-IV-TR criteria for primary insomnia.
Interventions: Nightly zolpidem, 10 mg (5 mg for patients > 60 yrs) or placebo 30 minutes before bedtime for 8 months.
Measurements and results: Polysomnographic sleep parameters and morning subject assessments of sleep on 2 nights in months 1 and 8. Relative to placebo, zolpidem significantly increased overall total sleep time and sleep efficiency, reduced sleep latency and wake after sleep onset when assessed at months 1 and 8. Overall, subjective evaluations of efficacy were not shown among treatment groups.
Conclusions: In adults with primary insomnia, nightly zolpidem administration remained efficacious across 8 months of nightly use.
Clinical trial information: ClinicalTrials.gov Identifier: NCT01006525; Trial Name: Safety and Efficacy of Chronic Hypnotic Use; http://clinicaltrials.gov/ct2/show/NCT01006525.
Keywords: Primary insomnia; hypnotics; randomized controlled trial; zolpidem.
Figures
Similar articles
-
Gender Differences in the Efficacy and Safety of Chronic Nightly Zolpidem.J Clin Sleep Med. 2016 Mar;12(3):319-25. doi: 10.5664/jcsm.5574. J Clin Sleep Med. 2016. PMID: 26446253 Free PMC article. Clinical Trial.
-
Comparison of Lemborexant With Placebo and Zolpidem Tartrate Extended Release for the Treatment of Older Adults With Insomnia Disorder: A Phase 3 Randomized Clinical Trial.JAMA Netw Open. 2019 Dec 2;2(12):e1918254. doi: 10.1001/jamanetworkopen.2019.18254. JAMA Netw Open. 2019. PMID: 31880796 Free PMC article. Clinical Trial.
-
Efficacy and safety of zolpidem-MR: a double-blind, placebo-controlled study in adults with primary insomnia.Sleep Med. 2006 Aug;7(5):397-406. doi: 10.1016/j.sleep.2006.04.008. Epub 2006 Jul 3. Sleep Med. 2006. PMID: 16815744 Clinical Trial.
-
Efficacy and safety of Zolpidem in the treatment of insomnia disorder for one month: a meta-analysis of a randomized controlled trial.Sleep Med. 2021 Nov;87:250-256. doi: 10.1016/j.sleep.2021.09.005. Epub 2021 Sep 20. Sleep Med. 2021. PMID: 34688027 Review.
-
Sublingual zolpidem (Edluar™; Sublinox™).CNS Drugs. 2012 Nov;26(11):1003-10. doi: 10.1007/s40263-012-0009-y. CNS Drugs. 2012. PMID: 23034583 Review.
Cited by
-
Role of optimum diagnosis and treatment of insomnia in patients with hypertension and diabetes: A review.J Family Med Prim Care. 2018 Sep-Oct;7(5):876-883. doi: 10.4103/jfmpc.jfmpc_337_17. J Family Med Prim Care. 2018. PMID: 30598926 Free PMC article.
-
Insomnia as a path to alcoholism: tolerance development and dose escalation.Sleep. 2018 Aug 1;41(8):zsy091. doi: 10.1093/sleep/zsy091. Sleep. 2018. PMID: 29762764 Free PMC article.
-
Personalized transcranial alternating current stimulation improves sleep quality: Initial findings.Front Hum Neurosci. 2023 Jan 10;16:1066453. doi: 10.3389/fnhum.2022.1066453. eCollection 2022. Front Hum Neurosci. 2023. PMID: 36704097 Free PMC article.
-
Randomized controlled trial of zolpidem as a pharmacotherapy for cannabis use disorder.J Subst Use Addict Treat. 2024 Jan;156:209180. doi: 10.1016/j.josat.2023.209180. Epub 2023 Oct 5. J Subst Use Addict Treat. 2024. PMID: 37802317 Free PMC article. Clinical Trial.
-
Assessing the Risk of Bias in Randomized Clinical Trials With Large Language Models.JAMA Netw Open. 2024 May 1;7(5):e2412687. doi: 10.1001/jamanetworkopen.2024.12687. JAMA Netw Open. 2024. PMID: 38776081 Free PMC article.
References
-
- Sateia MJ, Doghramji K, Hauri PJ, Morin CM. Evaluation of chronic insomnia. Sleep. 2000;23:1–66. - PubMed
-
- Sleep; National Institutes of Health State of the Science Conference Statement on Manifestations and Management of Chronic Insomnia in Adults; June 13-15, 2005; 2005. pp. 1049–57. - PubMed
-
- Krakow B, Ulibarri VA, Romero EA. Persistent insomnia in chronic hypnotic users presenting to a sleep medical center: retrospective chart review of 137 consecutive patients. J Nerv Ment Dis. 2010;198:734–41. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical