Targeting the Large Subunit of Human Ribonucleotide Reductase for Cancer Chemotherapy
- PMID: 23115527
- PMCID: PMC3483043
- DOI: 10.3390/ph4101328
Targeting the Large Subunit of Human Ribonucleotide Reductase for Cancer Chemotherapy
Abstract
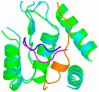
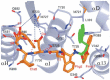
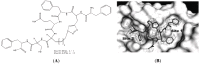
Ribonucleotide reductase (RR) is a crucial enzyme in de novo DNA synthesis, where it catalyses the rate determining step of dNTP synthesis. RRs consist of a large subunit called RR1 (α), that contains two allosteric sites and one catalytic site, and a small subunit called RR2 (β), which houses a tyrosyl free radical essential for initiating catalysis. The active form of mammalian RR is an α(n)β(m) hetero oligomer. RR inhibitors are cytotoxic to proliferating cancer cells. In this brief review we will discuss the three classes of RR, the catalytic mechanism of RR, the regulation of the dNTP pool, the substrate selection, the allosteric activation, inactivation by ATP and dATP, and the nucleoside drugs that target RR. We will also discuss possible strategies for developing a new class of drugs that disrupts the RR assembly.
Figures






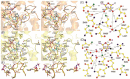

References
-
- Nordlund P., Reichard P. Ribonucleotide reductases. Annu. Rev. Biochem. 2006;75:681–706. - PubMed
-
- Kolberg M., Strand K.R., Graff P., Andersson K.K. Structure, function, and mechanism of ribonucleotide reductases. Biochim. Biophys. Acta. 2004;1699:1–34. - PubMed
-
- Bertani L.E., Haggmark A., Reichard P. Synthesis of pyrimidine deoxyribonucleoside diphosphates with enzymes from Escherichia coli. J. Biol. Chem. 1961;236:PC67–PC68. - PubMed
-
- Jordan A., Reichard P. Ribonucleotide reductases. Annu. Rev. Biochem. 1998;67:71–98. - PubMed
-
- Reichard P. From RNA to DNA, why so many ribonucleotide reductases? Science. 1993;260:1773–1777. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
