Multivitamins in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial
- PMID: 23117775
- PMCID: PMC3501249
- DOI: 10.1001/jama.2012.14805
Multivitamins in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial
Abstract
Context: Although multivitamins are used to prevent vitamin and mineral deficiency, there is a perception that multivitamins may prevent cardiovascular disease (CVD). Observational studies have shown inconsistent associations between regular multivitamin use and CVD, with no long-term clinical trials of multivitamin use.
Objective: To determine whether long-term multivitamin supplementation decreases the risk of major cardiovascular events among men.
Design, setting, and participants: The Physicians' Health Study II, a randomized, double-blind, placebo-controlled trial of a common daily multivitamin, began in 1997 with continued treatment and follow-up through June 1, 2011. A total of 14,641 male US physicians initially aged 50 years or older (mean, 64.3 [SD, 9.2] years), including 754 men with a history of CVD at randomization, were enrolled.
Intervention: Daily multivitamin or placebo.
Main outcome measures: Composite end point of major cardiovascular events, including nonfatal myocardial infarction (MI), nonfatal stroke, and CVD mortality. Secondary outcomes included MI and stroke individually.
Results: During a median follow-up of 11.2 (interquartile range, 10.7-13.3) years, there were 1732 confirmed major cardiovascular events. Compared with placebo, there was no significant effect of a daily multivitamin on major cardiovascular events (11.0 and 10.8 events per 1000 person-years for multivitamin vs placebo, respectively; hazard ratio [HR], 1.01; 95% CI, 0.91-1.10; P = .91). Further, a daily multivitamin had no effect on total MI (3.9 and 4.2 events per 1000 person-years; HR, 0.93; 95% CI, 0.80-1.09; P = .39), total stroke (4.1 and 3.9 events per 1000 person-years; HR, 1.06; 95% CI, 0.91-1.23; P = .48), or CVD mortality (5.0 and 5.1 events per 1000 person-years; HR, 0.95; 95% CI, 0.83-1.09; P = .47). A daily multivitamin was also not significantly associated with total mortality (HR, 0.94; 95% CI, 0.88-1.02; P = .13). The effect of a daily multivitamin on major cardiovascular events did not differ between men with or without a baseline history of CVD (P = .62 for interaction).
Conclusion: Among this population of US male physicians, taking a daily multivitamin did not reduce major cardiovascular events, MI, stroke, and CVD mortality after more than a decade of treatment and follow-up.
Trial registration: clinicaltrials.gov Identifier: NCT00270647.
Figures
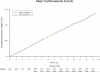



Comment in
-
Multivitamins in prevention of cardiovascular disease.JAMA. 2012 Nov 7;308(17):1802-3. doi: 10.1001/jama.2012.28259. JAMA. 2012. PMID: 23117781 No abstract available.
-
Prevention. Multivitamins do not reduce the risk of cardiovascular disease.Nat Rev Cardiol. 2013 Jan;10(1):7. doi: 10.1038/nrcardio.2012.175. Epub 2012 Nov 20. Nat Rev Cardiol. 2013. PMID: 23165066 No abstract available.
-
ACP Journal Club. Daily multivitamin supplements did not reduce risk for major CV events over > 10 years in men.Ann Intern Med. 2013 Feb 19;158(4):JC8. doi: 10.7326/0003-4819-158-4-201302190-02008. Ann Intern Med. 2013. PMID: 23420255 No abstract available.
-
Long-term supplementation with multivitamins and minerals did not improve male US physicians' cardiovascular health or prolong their lives.Evid Based Med. 2013 Dec;18(6):218-9. doi: 10.1136/eb-2012-101178. Epub 2013 Apr 12. Evid Based Med. 2013. PMID: 23585077 No abstract available.
References
-
- Timbo BB, Ross MP, McCarthy PV, Lin CT. Dietary supplements in a national survey: Prevalence of use and reports of adverse events. J Am Diet Assoc. 2006;106(12):1966–1974. - PubMed
-
- Ervin RB, Wright JD, Kennedy-Stephenson J. Use of dietary supplements in the United States, 1988–94. 244. Vol. 11. National Center for Health Statistics; Washington DC: 1999. pp. i–14. Vital Health Stat. - PubMed
-
- Blendon RJ, DesRoches CM, Benson JM, Brodie M, Altman DE. Americans' views on the use and regulation of dietary supplements. Arch Intern Med. 2001;161(6):805–810. - PubMed
-
- Gahche J, Bailey R, Burt V, et al. Dietary supplement use among U.S. adults has increased since NHANES III (1988–1994) NCHS Data Brief. 2011;(61):1–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

