Combing signals from spontaneous reports and electronic health records for detection of adverse drug reactions
- PMID: 23118093
- PMCID: PMC3628045
- DOI: 10.1136/amiajnl-2012-000930
Combing signals from spontaneous reports and electronic health records for detection of adverse drug reactions
Abstract
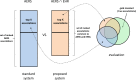
Objective: Data-mining algorithms that can produce accurate signals of potentially novel adverse drug reactions (ADRs) are a central component of pharmacovigilance. We propose a signal-detection strategy that combines the adverse event reporting system (AERS) of the Food and Drug Administration and electronic health records (EHRs) by requiring signaling in both sources. We claim that this approach leads to improved accuracy of signal detection when the goal is to produce a highly selective ranked set of candidate ADRs.
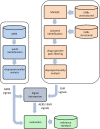
Materials and methods: Our investigation was based on over 4 million AERS reports and information extracted from 1.2 million EHR narratives. Well-established methodologies were used to generate signals from each source. The study focused on ADRs related to three high-profile serious adverse reactions. A reference standard of over 600 established and plausible ADRs was created and used to evaluate the proposed approach against a comparator.
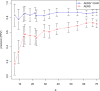
Results: The combined signaling system achieved a statistically significant large improvement over AERS (baseline) in the precision of top ranked signals. The average improvement ranged from 31% to almost threefold for different evaluation categories. Using this system, we identified a new association between the agent, rasburicase, and the adverse event, acute pancreatitis, which was supported by clinical review.
Conclusions: The results provide promising initial evidence that combining AERS with EHRs via the framework of replicated signaling can improve the accuracy of signal detection for certain operating scenarios. The use of additional EHR data is required to further evaluate the capacity and limits of this system and to extend the generalizability of these results.
Figures
References
-
- Schneeweiss S, Hasford J, Gottler M, et al. Admissions caused by adverse drug events to internal medicine and emergency departments in hospitals: a longitudinal population-based study. Eur J Clin Pharmacol 2002;58:285–91 - PubMed
-
- Classen DC, Pestotnik SL, Evans RS, et al. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA 1997;277:301–6 - PubMed
-
- Bates DW, Spell N, Cullen DJ, et al. The costs of adverse drug events in hospitalized patients. Adverse Drug Events Prevention Study Group. JAMA 1997;277:307–11 - PubMed
-
- Adverse Event Reporting System http://www.fda.gov/cder/aers/default.htm (accessed Jul 2007).
-
- Szarfman A, Machado SG, O'Neill RT. Use of screening algorithms and computer systems to efficiently signal higher-than-expected combinations of drugs and events in the US FDA's spontaneous reports database. Drug Saf 2002;25:381–92 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

