Effects of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D-conjugate vaccine on nasopharyngeal bacterial colonization in young children: a randomized controlled trial
- PMID: 23118268
- PMCID: PMC3540043
- DOI: 10.1093/cid/cis922
Effects of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D-conjugate vaccine on nasopharyngeal bacterial colonization in young children: a randomized controlled trial
Abstract
Background: This study evaluated the effects of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D-conjugate vaccine (PHiD-CV) on nasopharyngeal bacterial colonization compared with the 7-valent pneumococcal conjugate vaccine (7vCRM) in young children.
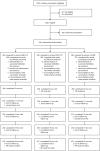
Methods: A randomized controlled trial in the Netherlands, initiated 2 years after 7vCRM introduction, was conducted between 1 April 2008 and 1 December 2010. Infants (N = 780) received either PHiD-CV or 7vCRM (2:1) at 2, 3, 4, and 11-13 months of age. Nasopharyngeal samples taken at 5, 11, 14, 18, and 24 months of age were cultured to detect Haemophilus influenzae, Streptococcus pneumoniae, Moraxella catarrhalis, and Staphylococcus aureus. Polymerase chain reaction assays quantified H. influenzae and S. pneumoniae and confirmed H. influenzae as nontypeable (NTHi). Primary outcome measure was vaccine efficacy (VE) against NTHi colonization.
Results: In both groups, NTHi colonization increased with age from 33% in 5-month-olds to 65% in 24-month-olds. Three months postbooster, VE against colonization was 0.5% (95% confidence interval [CI], -21.8% to 18.4%) and VE against acquisition 10.9% (95% CI, -31.3% to 38.9%). At each sampling moment, no differences between groups in either NTHi prevalence or H. influenzae density were detected. Streptococcus pneumoniae (range, 39%-57%), M. catarrhalis (range, 63%--69%), and S. aureus (range, 9%-30%) colonization patterns were similar between groups.
Conclusions: PHiD-CV had no differential effect on nasopharyngeal NTHi colonization or H. influenzae density in healthy Dutch children up to 2 years of age, implying that herd effects for NTHi are not to be expected. Other bacterial colonization patterns were also similar.
Trial registration: ClinicalTrials.gov NCT00652951.
Figures


References
-
- O'Brien KL, Wolfson LJ, Watt JP, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. - PubMed
-
- Rovers MM, Schilder AGM, Zielhuis GA, Rosenfeld RM. Otitis media. Lancet. 2004;363:465–73. - PubMed
-
- Murphy TF, Faden H, Bakaletz LO, et al. Nontypeable Haemophilus influenzae as a pathogen in children. Pediatr Infect Dis J. 2009;28:43–8. - PubMed
-
- Leibovitz E, Jacobs MR, Dagan R. Haemophilus influenzae: a significant pathogen in acute otitis media. Pediatr Infect Dis J. 2004;23:1142–52. - PubMed
-
- Barkai G, Leibovitz E, Givon Lavi N, Dagan R. Potential contribution by nontypable Haemophilus influenzae in protracted and recurrent acute otitis media. Pediatr Infect Dis J. 2009;28:466–71. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

