Enhancement of the dissolution rate and bioavailability of fenofibrate by a melt-adsorption method using supercritical carbon dioxide
- PMID: 23118538
- PMCID: PMC3484728
- DOI: 10.2147/IJN.S36939
Enhancement of the dissolution rate and bioavailability of fenofibrate by a melt-adsorption method using supercritical carbon dioxide
Abstract
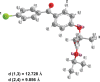
Background: The aim of this study was to enhance the bioavailability of fenofibrate, a poorly water-soluble drug, using a melt-adsorption method with supercritical CO(2).
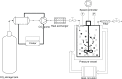
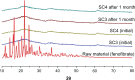
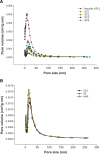
Methods: Fenofibrate was loaded onto Neusilin(®) UFL2 at different weight ratios of fenofibrate to Neusilin UFL2 by melt-adsorption using supercritical CO(2). For comparison, fenofibrate-loaded Neusilin UFL2 was prepared by solvent evaporation and hot melt-adsorption methods. The fenofibrate formulations prepared were characterized by differential scanning calorimetry, powder x-ray diffractometry, specific surface area, pore size distribution, scanning electron microscopy, and energy-dispersive x-ray spectrometry. In vitro dissolution and in vivo bioavailability were also investigated.
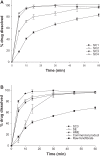
Results: Fenofibrate was distributed into the pores of Neusilin UFL2 and showed reduced crystal formation following adsorption. Supercritical CO(2) facilitated the introduction of fenofibrate into the pores of Neusilin UFL2. Compared with raw fenofibrate, fenofibrate from the prepared powders showed a significantly increased dissolution rate and better bioavailability. In particular, the area under the drug concentration-time curve and maximal serum concentration of the powders prepared using supercritical CO(2) were 4.62-fold and 4.52-fold greater than the corresponding values for raw fenofibrate.
Conclusion: The results of this study highlight the usefulness of the melt-adsorption method using supercritical CO(2) for improving the bioavailability of fenofibrate.
Keywords: bioavailability; fenofibrate; melt adsorption; supercritical CO2.
Figures





Similar articles
-
Melt Amorphisation of Orlistat with Mesoporous Silica Using a Supercritical Carbon Dioxide: Effects of Pressure, Temperature, and Drug Loading Ratio and Comparison with Other Conventional Amorphisation Methods.Pharmaceutics. 2020 Apr 20;12(4):377. doi: 10.3390/pharmaceutics12040377. Pharmaceutics. 2020. PMID: 32326103 Free PMC article.
-
Dissolution-rate enhancement of fenofibrate by adsorption onto silica using supercritical carbon dioxide.Int J Pharm. 2008 Aug 6;360(1-2):213-8. doi: 10.1016/j.ijpharm.2008.04.041. Epub 2008 May 4. Int J Pharm. 2008. PMID: 18550302
-
Effect of magnesium carbonate on the solubility, dissolution and oral bioavailability of fenofibric acid powder as an alkalising solubilizer.Arch Pharm Res. 2016 Apr;39(4):531-538. doi: 10.1007/s12272-015-0701-9. Epub 2016 Mar 18. Arch Pharm Res. 2016. PMID: 26992922
-
Total allowable concentrations of monomeric inorganic aluminum and hydrated aluminum silicates in drinking water.Crit Rev Toxicol. 2012 May;42(5):358-442. doi: 10.3109/10408444.2012.674101. Crit Rev Toxicol. 2012. PMID: 22512666 Review.
-
Application of supercritical fluid technology for solid dispersion to enhance solubility and bioavailability of poorly water-soluble drugs.Int J Pharm. 2021 Dec 15;610:121247. doi: 10.1016/j.ijpharm.2021.121247. Epub 2021 Nov 2. Int J Pharm. 2021. PMID: 34740762 Review.
Cited by
-
Development of sustained-release microparticles containing tamsulosin HCl for orally disintegrating tablet using melt-adsorption method.Drug Deliv Transl Res. 2018 Jun;8(3):552-564. doi: 10.1007/s13346-018-0477-9. Drug Deliv Transl Res. 2018. PMID: 29359246
-
Preparation and Characterization of Fenofibrate Microparticles with Surface-Active Additives: Application of a Supercritical Fluid-Assisted Spray-Drying Process.Pharmaceutics. 2021 Dec 2;13(12):2061. doi: 10.3390/pharmaceutics13122061. Pharmaceutics. 2021. PMID: 34959341 Free PMC article.
-
Fabrication and evaluation of valsartan-polymer- surfactant composite nanoparticles by using the supercritical antisolvent process.Int J Nanomedicine. 2014 Nov 7;9:5167-76. doi: 10.2147/IJN.S71891. eCollection 2014. Int J Nanomedicine. 2014. PMID: 25404856 Free PMC article.
-
Pharmaceutical Characterization and In Vivo Evaluation of Orlistat Formulations Prepared by the Supercritical Melt-Adsorption Method Using Carbon Dioxide: Effects of Mesoporous Silica Type.Pharmaceutics. 2020 Apr 8;12(4):333. doi: 10.3390/pharmaceutics12040333. Pharmaceutics. 2020. PMID: 32276311 Free PMC article.
-
A One-Step Twin-Screw Melt Granulation with Gelucire 48/16 and Surface Adsorbent to Improve the Solubility of Poorly Soluble Drugs: Effect of Formulation Variables on Dissolution and Stability.AAPS PharmSciTech. 2021 Feb 19;22(3):79. doi: 10.1208/s12249-021-01945-8. AAPS PharmSciTech. 2021. PMID: 33606113
References
-
- Adkins JC, Faulds D. Micronised fenofibrate: a review of its pharmacodynamic properties and clinical efficacy in the management of dyslipidemia. Drugs. 1997;54:615–633. - PubMed
-
- Murakami H, Murakami R, Kambe F, et al. Fenofibrate activates AMPK and increases eNOS phosphorylation in HUVEC. Biochem Biophy Res Commun. 2006;341:973–978. - PubMed
-
- Jinno J, Kamada N, Miyake M, et al. Effect of particle size reduction on dissolution and oral absorption of a poorly water-soluble drug, cilostazol, in beagle dogs. J Control Release. 2006;111:56–64. - PubMed
-
- Hancock BC, Zografi G. Characteristics and significance of the amorphous state in pharmaceutical systems. J Pharm Sci. 1997;86:1–12. - PubMed
-
- Van Nijlen T, Brennan K, Van den Mooter G, Blaton N, Kinget R, Augustijns P. Improvement of the dissolution rate of artemisinin by means of supercritical fluid technology and solid dispersions. Int J Pharm. 2003;254:173–181. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

