Effect of prenatal administration of venlafaxine on postnatal development of rat offspring
- PMID: 23118594
- PMCID: PMC3485660
- DOI: 10.2478/v10102-012-0016-3
Effect of prenatal administration of venlafaxine on postnatal development of rat offspring
Abstract
About 3% of pregnant women are treated with antidepressant drugs during gestation. After delivery the number of treated women increases to 5 to 7%. Most prescribed antidepressants in pregnancy are selective serotonin re-uptake inhibitors and/or serotonin and noradrenaline re-uptake inhibitors, such as fluoxetine, paroxetine, sertraline, citalopram and venlafaxine (VENF). Despite the fact that VENF has been assigned to pregnancy category C by the FDA, experimental studies with this drug are rare. The aim of this pilot study was to investigate the effect of prenatal administration of VENF on early postnatal development of rat offspring and selected biochemical variables at weaning of pups. Pregnant female Wistar rats were treated with VENF from day 15 to 20 of gestation at the doses of 7.5, 37.5 and 70 mg/kg. Females were allowed to spontaneously deliver their pups. After delivery the pups were inspected for viability, gross malformation and they were weighed on day 0, 4 and 21 post partum. On day 21 post partum, the pups were killed, brains were removed from the skulls and blood samples were collected for biochemical assay (proteins, glucose-GOD, glucose-HEX, lactate dehydrogenase, aspartate aminotransferase, alanine aminotransferase and total antioxidant status). The study showed that prenatal VENF administration resulted in a mild maternal intoxication manifested by decreased body weight gain of pregnant females. There was no effect of the drug tested on the body and brain weights of offspring. No obvious morphological alterations were observed in the delivered pups. Similarly, there were no changes in the selected biochemical variables determined.
Keywords: fetus; noradrenaline re-uptake inhibitors; postnatal development; pregnancy; pups; rat; selective serotonin re-uptake inhibitors and/or serotonin; venlafaxine.
Figures
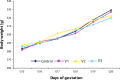
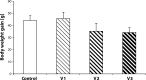




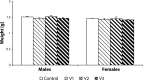
Similar articles
-
NTP technical report on the toxicity studies of Dibutyl Phthalate (CAS No. 84-74-2) Administered in Feed to F344/N Rats and B6C3F1 Mice.Toxic Rep Ser. 1995 Apr;30:1-G5. Toxic Rep Ser. 1995. PMID: 12209194
-
Postnatal development of rats exposed to fluoxetine or venlafaxine during the third week of pregnancy.Braz J Med Biol Res. 1999 Jan;32(1):93-8. doi: 10.1590/s0100-879x1999000100014. Braz J Med Biol Res. 1999. PMID: 10347775
-
Effect of Selected Antidepressants on Placental Homeostasis of Serotonin: Maternal and Fetal Perspectives.Pharmaceutics. 2021 Aug 20;13(8):1306. doi: 10.3390/pharmaceutics13081306. Pharmaceutics. 2021. PMID: 34452265 Free PMC article.
-
In utero exposure to venlafaxine, a serotonin-norepinephrine reuptake inhibitor, increases cardiac anomalies and alters placental and heart serotonin signaling in the rat.Birth Defects Res A Clin Mol Teratol. 2016 Dec;106(12):1044-1055. doi: 10.1002/bdra.23537. Epub 2016 Jul 7. Birth Defects Res A Clin Mol Teratol. 2016. PMID: 27384265
-
Neuroendocrine and behavioral consequences of untreated and treated depression in pregnancy and lactation.Neuro Endocrinol Lett. 2014;35 Suppl 2:169-74. Neuro Endocrinol Lett. 2014. PMID: 25638382 Review.
Cited by
-
Prenatal Venlafaxine Exposure-Induced Neurocytoarchitectural and Neuroapoptotic Degeneration in Striatum and Hippocampus of Developing Fetal Brain, Manifesting Long-term Neurocognitive Impairments in Rat Offspring.Neurotox Res. 2022 Oct;40(5):1174-1190. doi: 10.1007/s12640-022-00541-3. Epub 2022 Jul 12. Neurotox Res. 2022. PMID: 35819590
-
Effects of Venlafaxine on the Size of Brain and Expression of SHANK3, TUBB5 and DDC Genes in BALB/c Mice.Psychopharmacol Bull. 2023 Aug 11;53(3):22-34. Psychopharmacol Bull. 2023. PMID: 37601086 Free PMC article.
-
Prevalence of Selective Serotonin Reuptake Inhibitor Use Among Pregnant Women From 2017 to 2020 in King Abdulaziz Medical City, Jeddah, Saudi Arabia: A Retrospective Study.Cureus. 2023 Oct 26;15(10):e47745. doi: 10.7759/cureus.47745. eCollection 2023 Oct. Cureus. 2023. PMID: 38021702 Free PMC article.
-
Imipramine and Venlafaxine Differentially Affect Primary Glial Cultures of Prenatally Stressed Rats.Front Pharmacol. 2020 Jan 31;10:1687. doi: 10.3389/fphar.2019.01687. eCollection 2019. Front Pharmacol. 2020. PMID: 32076407 Free PMC article.
-
Risks of using SSRI / SNRI antidepressants during pregnancy and lactation.Interdiscip Toxicol. 2017 Sep;10(1):30-34. doi: 10.1515/intox-2017-0004. Interdiscip Toxicol. 2017. PMID: 30123033 Free PMC article. Review.
References
-
- Bellantuono C, Migliarese G, Maggioni F, Imperadore G. Anridepressant drugs and breastfeeding. Recent Prog Med. 2007;98:29–42. - PubMed
-
- Chung TK, Lau TK, Yip AS, Chiu HF, Lee DT. Antepartum depressive symptomatology is associated with adverse obstetric and neonatal outcomes. Psychosom Med. 2001;63:830–834. - PubMed
-
- Da-Silva VA, Alterburg SP, Malheiros LR, Thomaz TG, Lindsey CJ. Postnatal development of rats exposed to fluoxetine or venlafaxine during the third week of pregnancy. Braz J Med Biol Res. 1999;32:93–98. - PubMed
-
- DeSantis DT, Schmaltz LW. The mother-litter relationship in developmental rat studies: cannibalism vs caring. Dev Psychobiol. 1984;17:255–62. - PubMed
LinkOut - more resources
Full Text Sources
