Management of patients with cystic fibrosis and allergic bronchopulmonary aspergillosis using anti-immunoglobulin e therapy (omalizumab)
- PMID: 23118662
- PMCID: PMC3428193
- DOI: 10.5863/1551-6776-17.1.88
Management of patients with cystic fibrosis and allergic bronchopulmonary aspergillosis using anti-immunoglobulin e therapy (omalizumab)
Abstract
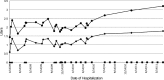
Omalizumab is a recombinant DNA-derived humanized immunoglobulin G (IgG) anti-IgE monoclonal antibody approved for use in patients with allergic asthma. However, it is not approved for allergic bronchopulmonary aspergillosis (ABPA). Conflicting reports exist about the effects of omalizumab on ABPA in patients with cystic fibrosis (CF). We report 2 patients with CF treated with omalizumab, in whom frequency of ABPA exacerbations was markedly reduced with treatment. Additionally, hospitalizations were reduced from 5 per year to once in 18 months in the first patient and from twice to once per year in the second patient. Free IgE decreased by 87.9% after 6 months of therapy in the first patient and by 95.6% after 7 months of therapy in the second patient. Neither of the two patients had evidence of asthma. Omalizumab may be useful in treating ABPA in patients with CF, and including free IgE in monitoring the response to therapy will be helpful.
Keywords: Allergic bronchopulmonary aspergillosis; cystic fibrosis; free IgE; omalizumab.
Figures


References
-
- Stevens DA, Moss RB, Kurup VP, et al. Allergic bronchopulmonary aspergillosis in cystic fibrosis–state of the art: Cystic Fibrosis Foundation Consensus Conference. Clin Infect Dis. 2003;37(suppl 3):S225–S264. - PubMed
-
- Kanu A, Patel K. Treatment of allergic bronchopulmonary aspergillosis (ABPA) in CF with anti-IgE antibody (omalizumab) Pediatr Pulmonol. 2008;43(12):1249–1251. - PubMed
-
- Zirbes JM, Milla CE. Steroid-sparing effect of omalizumab for allergic bronchopulmonary aspergillosis and cystic fibrosis. Pediatr Pulmonol. 2008;43(6):607–610. - PubMed
-
- Lebecque P, Leonard A, Pilette C. Omalizumab for treatment of ASPA exacerbations in CF patients. Pediatr Pulmonol. 2009;44(5):516. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
