Feasibility of short-term infusion of magnesium sulfate in pediatric patients with status asthmaticus
- PMID: 23118666
- PMCID: PMC3470434
- DOI: 10.5863/1551-6776-17.2.150
Feasibility of short-term infusion of magnesium sulfate in pediatric patients with status asthmaticus
Abstract
Objective: This report describes the feasibility of high-dose magnesium sulfate infusion in pediatric patients with status asthmaticus.
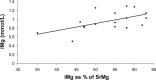
Methods: Retrospective chart review over a 3-year period of all patients younger than 18 years of age with status asthmaticus who underwent a high-dose magnesium sulfate infusion for 4 hours. All patients were breathing spontaneously but were refractory to conventional therapy. The magnesium sulfate infusion regimen was 50 mg/kg (for patients weighing >30 kg) or 75 mg/kg (for those weighing ≤30 kg) over a period of 30 to 45 minutes, followed by a continuous infusion of 40 mg/kg/hr for 4 hours. Information regarding vital and clinical respiratory signs, serum magnesium (SrMg), ionized magnesium (iMg), electrocardiograms, and cardiac troponin levels were retrieved. We analyzed the relationship between SrMg and iMg by using linear regression analysis.
Results: Nineteen patients were included. At the end of the infusion, SrMg levels were 4.4 ± 0.8 mg/dL, and iMg levels were 0.95 ± 0.2 mmol/L. SrMg levels only moderately predicted iMg (r(2) = 0.541). There were no reports of hypotension, respiratory failure, neurological problems, or nausea. Discomfort at the site of infusion was reported in three cases. Troponin levels (n = 12) and electrocardiograms (n = 12), when available, were noted at the end of the infusion and were normal in all patients p=0.01.
Conclusions: In this case series, short-term high-dose administration of magnesium sulfate in the context of status asthmaticus was feasible, and we did not observe clinical complications with its use. Total SrMg was inadequate to reflect the active form of magnesium, iMg. The dose used achieved theoretical therapeutic levels of iMg.
Keywords: asthma; feasibility; infusion; magnesium sulfate; status asthmaticus.
Figures
Similar articles
-
High-dose magnesium sulfate infusion protocol for status asthmaticus: a safety and pharmacokinetics cohort study.Intensive Care Med. 2013 Jan;39(1):117-22. doi: 10.1007/s00134-012-2734-6. Epub 2012 Nov 6. Intensive Care Med. 2013. PMID: 23129148 Clinical Trial.
-
Continuous Magnesium Sulfate Infusions for Status Asthmaticus in Children: A Systematic Review.Front Pediatr. 2022 Mar 22;10:853574. doi: 10.3389/fped.2022.853574. eCollection 2022. Front Pediatr. 2022. PMID: 35391743 Free PMC article.
-
Comparison of Two High-Dose Magnesium Infusion Regimens in the Treatment of Status Asthmaticus.J Pediatr Pharmacol Ther. 2016 May-Jun;21(3):233-8. doi: 10.5863/1551-6776-21.3.233. J Pediatr Pharmacol Ther. 2016. PMID: 27453701 Free PMC article.
-
Safety of prolonged magnesium sulfate infusions during treatment for severe pediatric status asthmaticus.Pediatr Pulmonol. 2019 Dec;54(12):1941-1947. doi: 10.1002/ppul.24499. Epub 2019 Sep 3. Pediatr Pulmonol. 2019. PMID: 31478612
-
Rapid infusion of magnesium sulfate obviates need for intubation in status asthmaticus.Am J Emerg Med. 1994 Mar;12(2):164-6. doi: 10.1016/0735-6757(94)90238-0. Am J Emerg Med. 1994. PMID: 8161388 Review.
Cited by
-
Efficacy and Safety of Prolonged Magnesium Sulfate Infusions in Children With Refractory Status Asthmaticus.Front Pediatr. 2022 Jun 9;10:860921. doi: 10.3389/fped.2022.860921. eCollection 2022. Front Pediatr. 2022. PMID: 35757130 Free PMC article.
-
High-dose magnesium sulfate infusion protocol for status asthmaticus: a safety and pharmacokinetics cohort study.Intensive Care Med. 2013 Jan;39(1):117-22. doi: 10.1007/s00134-012-2734-6. Epub 2012 Nov 6. Intensive Care Med. 2013. PMID: 23129148 Clinical Trial.
-
Continuous Magnesium Sulfate Infusions for Status Asthmaticus in Children: A Systematic Review.Front Pediatr. 2022 Mar 22;10:853574. doi: 10.3389/fped.2022.853574. eCollection 2022. Front Pediatr. 2022. PMID: 35391743 Free PMC article.
-
Prospective study of serum and ionized magnesium pharmacokinetics in the treatment of children with severe acute asthma.Eur J Clin Pharmacol. 2019 Jan;75(1):59-66. doi: 10.1007/s00228-018-2557-7. Epub 2018 Sep 26. Eur J Clin Pharmacol. 2019. PMID: 30259065
-
Stating the obvious: intravenous magnesium sulphate should be the first parenteral bronchodilator in paediatric asthma exacerbations unresponsive to first-line therapy.Breathe (Sheff). 2021 Dec;17(4):210113. doi: 10.1183/20734735.0113-2021. Breathe (Sheff). 2021. PMID: 35035570 Free PMC article. Review.
References
-
- Rodrigo G, Rodrigo K, Burschtin O. Efficacy of magnesium sulfate in acute adult-asthma: a meta-analysis of randomized trials. Am J Emerg Med. 2000;18(2):216–221. - PubMed
-
- Aali BS, Khazaeli P, Ghasemi F. Ionized and total magnesium concentration in patients with severe preeclampsia-eclampsia undergoing magnesium sulfate therapy. J Obstet Gynaecol Res. 2007;33(2):138–143. - PubMed
-
- Raimondi F, Migliaro F, Capasso L, et al. Intravenous magnesium sulphate vs. inhaled nitric oxide for moderate, persistent pulmonary hypertension of the newborn. A multicentre, retrospective study. J Trop Pediatr. 2008;54(3):196–199. - PubMed
LinkOut - more resources
Full Text Sources