GFP's mechanical intermediate states
- PMID: 23118864
- PMCID: PMC3485268
- DOI: 10.1371/journal.pone.0046962
GFP's mechanical intermediate states
Abstract
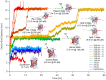
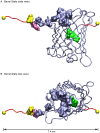
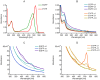
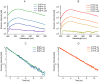
Green fluorescent protein (GFP) mutants have become the most widely used fluorescence markers in the life sciences, and although they are becoming increasingly popular as mechanical force or strain probes, there is little direct information on how their fluorescence changes when mechanically stretched. Here we derive high-resolution structural models of the mechanical intermediate states of stretched GFP using steered molecular dynamics (SMD) simulations. These structures were used to produce mutants of EGFP and EYFP that mimic GFP's different mechanical intermediates. A spectroscopic analysis revealed that a population of EGFP molecules with a missing N-terminal α-helix was significantly dimmed, while the fluorescence lifetime characteristic of the anionic chromophore state remained unaffected. This suggests a mechanism how N-terminal deletions can switch the protonation state of the chromophore, and how the fluorescence of GFP molecules in response to mechanical disturbance might be turned off.
Conflict of interest statement
Figures



References
-
- Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC (1994) Green fluorescent protein as a marker for gene expression. Science 263: 802–805. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

