Mechanisms of xenogeneic baboon platelet aggregation and phagocytosis by porcine liver sinusoidal endothelial cells
- PMID: 23118867
- PMCID: PMC3484054
- DOI: 10.1371/journal.pone.0047273
Mechanisms of xenogeneic baboon platelet aggregation and phagocytosis by porcine liver sinusoidal endothelial cells
Abstract
Background: Baboons receiving xenogeneic livers from wild type and transgenic pigs survive less than 10 days. One of the major issues is the early development of profound thrombocytopenia that results in fatal hemorrhage. Histological examination of xenotransplanted livers has shown baboon platelet activation, phagocytosis and sequestration within the sinusoids. In order to study the mechanisms of platelet consumption in liver xenotransplantation, we have developed an in vitro system to examine the interaction between pig endothelial cells with baboon platelets and to thereby identify molecular mechanisms and therapies.
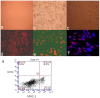
Methods: Fresh pig hepatocytes, liver sinusoidal and aortic endothelial cells were isolated by collagenase digestion of livers and processing of aortae from GTKO and Gal+ MGH-miniature swine. These primary cell cultures were then tested for the differential ability to induce baboon or pig platelet aggregation. Phagocytosis was evaluated by direct observation of CFSE labeled-platelets, which are incubated with endothelial cells under confocal light microscopy. Aurintricarboxylic acid (GpIb antagonist blocking interactions with von Willebrand factor/vWF), eptifibatide (Gp IIb/IIIa antagonist), and anti-Mac-1 Ab (anti-α(M)β(2) integrin Ab) were tested for the ability to inhibit phagocytosis.
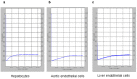
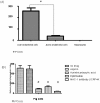
Results: None of the pig cells induced aggregation or phagocytosis of porcine platelets. However, pig hepatocytes, liver sinusoidal and aortic endothelial cells (GTKO and Gal+) all induced moderate aggregation of baboon platelets. Importantly, pig liver sinusoidal endothelial cells efficiently phagocytosed baboon platelets, while pig aortic endothelial cells and hepatocytes had minimal effects on platelet numbers. Anti-MAC-1 Ab, aurintricarboxylic acid or eptifibatide, significantly decreased baboon platelet phagocytosis by pig liver endothelial cells (P<0.01).
Conclusions: Although pig hepatocytes and aortic endothelial cells directly caused aggregation of baboon platelets, only pig liver endothelial cells efficiently phagocytosed baboon platelets. Blocking vWF and integrin adhesion pathways prevented both aggregation and phagocytosis.
Conflict of interest statement
Figures


References
-
- Cooper DK, Dorling A, Pierson RN III, Rees M, Seebach J, et al. (2007) a 1,3-galactosyltransferase gene knockout pigs for xenotransplantation: Where do we go from here? Transplantation 84: 1–7. - PubMed
-
- Loveland BE, Milland J, Kyriakou P, Thorley BR, Christiansen D, et al. (2004) Characterization of a CD46 transgenic pig and protection of transgenic kidneys against hyperacute rejection in non-immunosuppressed baboons. Xenotransplantation 11: 171–183. - PubMed
-
- Hara H, Long C, Lin YJ, Tai HC, Ezzelarab M, et al. (2008) In vitro investigation of pig cells for resistance to human antibody mediated rejection. Transpl Int 12: 1163–1174. - PubMed
-
- McGregor CG, Davies WR, Oi K, Teotia SS, Schirmer JM, et al. (2005) Cardiac xenotransplantation: Recent preclinical progress with 3-month median survival. J Thorac Cardiovasc Surg 130: 844–851. - PubMed
-
- Cozzi E, Bhatti F, Schmoeckel M, Chavez G, Smith KG, et al. (2000) Long-termsurvival of nonhuman primates receiving life supporting transgenic porcine kidney xenografts. Transplantation 70: 15–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

