Cellular and molecular effect of MEHP Involving LXRα in human fetal testis and ovary
- PMID: 23118965
- PMCID: PMC3484128
- DOI: 10.1371/journal.pone.0048266
Cellular and molecular effect of MEHP Involving LXRα in human fetal testis and ovary
Abstract
Background: Phthalates have been shown to have reprotoxic effects in rodents and human during fetal life. Previous studies indicate that some members of the nuclear receptor (NR) superfamilly potentially mediate phthalate effects. This study aimed to assess if expression of these nuclear receptors are modulated in the response to MEHP exposure on the human fetal gonads in vitro.
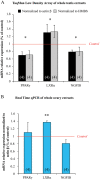
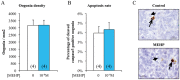
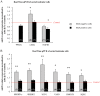
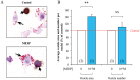
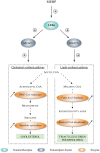
Methodology/principal findings: Testes and ovaries from 7 to 12 gestational weeks human fetuses were exposed to 10(-4)M MEHP for 72 h in vitro. Transcriptional level of NRs and of downstream genes was then investigated using TLDA (TaqMan Low Density Array) and qPCR approaches. To determine whether somatic or germ cells of the testis are involved in the response to MEHP exposure, we developed a highly efficient cytometric germ cell sorting approach. In vitro exposure of fetal testes and ovaries to MEHP up-regulated the expression of LXRα, SREBP members and of downstream genes involved in the lipid and cholesterol synthesis in the whole gonad. In sorted testicular cells, this effect is only observable in somatic cells but not in the gonocytes. Moreover, the germ cell loss induced by MEHP exposure, that we previously described, is restricted to the male gonad as oogonia density is not affected in vitro.
Conclusions/significance: We evidenced for the first time that phthalate increases the levels of mRNA for LXRα, and SREBP members potentially deregulating lipids/cholesterol synthesis in human fetal gonads. Interestingly, this novel effect is observable in both male and female whereas the germ cell apoptosis is restricted to the male gonad. Furthermore, we presented here a novel and potentially very useful flow cytometric cell sorting method to analyse molecular changes in germ cells versus somatic cells.
Conflict of interest statement
Figures


References
-
- Heudorf U, Mersch-Sundermann V, Angerer J (2007) Phthalates: toxicology and exposure. Int J Hyg Environ Health 210: 623–634. - PubMed
-
- Koo HJ, Lee BM (2004) Estimated exposure to phthaiates in cosmetics and risk assessment. J Toxicol Environ Health A 67: 1901–1914. - PubMed
-
- Kavlock R, Boekelheide K, Chapin R, Cunningham M, Faustman E, et al. (2002) NTP Center for the Evaluation of Risks to Human Reproduction: phthalates expert panel report on the reproductive and developmental toxicity of di-n-butyl phthalate. Reprod Toxicol 16: 489–527. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

