A T cell-inducing influenza vaccine for the elderly: safety and immunogenicity of MVA-NP+M1 in adults aged over 50 years
- PMID: 23118984
- PMCID: PMC3485192
- DOI: 10.1371/journal.pone.0048322
A T cell-inducing influenza vaccine for the elderly: safety and immunogenicity of MVA-NP+M1 in adults aged over 50 years
Abstract
Background: Current influenza vaccines have reduced immunogenicity and are of uncertain efficacy in older adults. We assessed the safety and immunogenicity of MVA-NP+M1, a viral-vectored influenza vaccine designed to boost memory T cell responses, in a group of older adults.
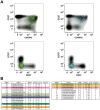
Methods: Thirty volunteers (aged 50-85) received a single intramuscular injection of MVA-NP+M1 at a dose of 1·5×10(8) plaque forming units (pfu). Safety and immunogenicity were assessed over a period of one year. The frequency of T cells specific for nucleoprotein (NP) and matrix protein 1 (M1) was determined by interferon-gamma (IFN-γ) ELISpot, and their phenotypic and functional properties were characterized by polychromatic flow cytometry. In a subset of M1-specific CD8(+) T cells, T cell receptor (TCR) gene expression was evaluated using an unbiased molecular approach.
Results: Vaccination with MVA-NP+M1 was well tolerated. ELISpot responses were boosted significantly above baseline following vaccination. Increases were detected in both CD4(+) and CD8(+) T cell subsets. Clonality studies indicated that MVA-NP+M1 expanded pre-existing memory CD8(+) T cells, which displayed a predominant CD27(+)CD45RO(+)CD57(-)CCR7(-) phenotype both before and after vaccination.
Conclusions: MVA-NP+M1 is safe and immunogenic in older adults. Unlike seasonal influenza vaccination, the immune responses generated by MVA-NP+M1 are similar between younger and older individuals. A T cell-inducing vaccine such as MVA-NP+M1 may therefore provide a way to circumvent the immunosenescence that impairs routine influenza vaccination.
Trial registration: ClinicalTrials.gov NCT00942071.
Conflict of interest statement
Figures

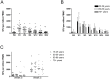



References
-
- Simonsen L, Taylor RJ, Viboud C, Miller MA, Jackson LA (2007) Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect Dis 7: 658–666. - PubMed
-
- Goodwin K, Viboud C, Simonsen L (2006) Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 24: 1159–1169. - PubMed
-
- Osterholm MT, Kelley NS, Sommer A, Belongia EA (2012) Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 12: 36–44. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

