Pericentromeric regions are refractory to prompt repair after replication stress-induced breakage in HPV16 E6E7-expressing epithelial cells
- PMID: 23119062
- PMCID: PMC3485353
- DOI: 10.1371/journal.pone.0048576
Pericentromeric regions are refractory to prompt repair after replication stress-induced breakage in HPV16 E6E7-expressing epithelial cells
Abstract
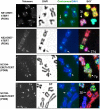
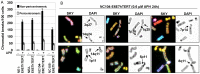
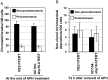
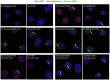
Chromosomal instability is the major form of genomic instability in cancer cells. Amongst various forms of chromosomal instability, pericentromeric or centromeric instability remains particularly poorly understood. In the present study, we found that pericentromeric instability, evidenced by dynamic formation of pericentromeric or centromeric rearrangements, breaks, deletions or iso-chromosomes, was a general phenomenon in human cells immortalized by expression of human papillomavirus type 16 E6 and E7 (HPV16 E6E7). In particular, for the first time, we surprisingly found a dramatic increase in the proportion of pericentromeric chromosomal aberrations relative to total aberrations in HPV16 E6E7-expressing cells 72 h after release from aphidicolin (APH)-induced replication stress, with pericentromeric chromosomal aberrations becoming the predominant type of structural aberrations (~70% of total aberrations). In contrast, pericentromeric aberrations accounted for only about 20% of total aberrations in cells at the end of APH treatment. This increase in relative proportion of pericentromeric aberrations after release from APH treatment revealed that pericentromeric breaks induced by replication stress are refractory to prompt repair in HPV16 E6E7-expressing epithelial cells. Telomerase-immortalized epithelial cells without HPV16 E6E7 expression did not exhibit such preferential pericentromeric instability after release from APH treatment. Cancer development is often associated with replication stress. Since HPV16 E6 and E7 inactivate p53 and Rb, and p53 and Rb pathway defects are common in cancer, our finding that pericentromeric regions are refractory to prompt repair after replication stress-induced breakage in HPV16 E6E7-expressing cells may shed light on mechanism of general pericentromeric instability in cancer.
Conflict of interest statement
Figures


References
-
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144: 646–674. - PubMed
-
- Lengauer C, Kinzler KW, Vogelstein B (1998) Genetic instabilities in human cancers. Nature 396: 643–649. - PubMed
-
- Negrini S, Gorgoulis VG, Halazonetis TD (2010) Genomic instability–an evolving hallmark of cancer. Nat Rev Mol Cell Biol 11: 220–228. - PubMed
-
- Jin Y, Mertens F, Jin C, Akervall J, Wennerberg J, et al. (1995) Nonrandom chromosome abnormalities in short-term cultured primary squamous cell carcinomas of the head and neck. Cancer Res 55: 3204–3210. - PubMed
-
- Jin Y, Jin C, Salemark L, Martins C, Wennerberg J, et al. (2000) Centromere cleavage is a mechanism underlying isochromosome formation in skin and head and neck carcinomas. Chromosoma 109: 476–481. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

