Is oxidized thioredoxin a major trigger for cysteine oxidation? Clues from a redox proteomics approach
- PMID: 23121505
- PMCID: PMC3613171
- DOI: 10.1089/ars.2012.5037
Is oxidized thioredoxin a major trigger for cysteine oxidation? Clues from a redox proteomics approach
Abstract
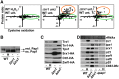
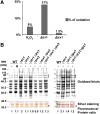
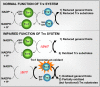
Cysteine oxidation mediates oxidative stress toxicity and signaling. It has been long proposed that the thioredoxin (Trx) system, which consists of Trx and thioredoxin reductase (Trr), is not only involved in recycling classical Trx substrates, such as ribonucleotide reductase, but it also regulates general cytoplasmic thiol homeostasis. To investigate such a role, we have performed a proteome-wide analysis of cells expressing or not the two components of the Trx system. We have compared the reversibly oxidized thiol proteomes of wild-type Schizosaccharomyces pombe cells with mutants lacking Trx or Trr. Specific Trx substrates are reversibly-oxidized in both strain backgrounds; however, in the absence of Trr, Trx can weakly recycle its substrates at the expense of an alternative electron donor. A massive thiol oxidation occurs only in cells lacking Trr, with 30% of all cysteine-containing peptides being reversibly oxidized; this oxidized cysteine proteome depends on the presence of Trxs. Our observations lead to the hypothesis that, in the absence of its reductase, the natural electron donor Trx becomes a powerful oxidant and triggers general thiol oxidation.
Figures


References
-
- Bulleid NJ. Ellgaard L. Multiple ways to make disulfides. Trends Biochem Sci. 2011;36:485–492. - PubMed
-
- Castillo EA. Ayte J. Chiva C. Moldon A. Carrascal M. Abian J. Jones N. Hidalgo E. Diethylmaleate activates the transcription factor Pap1 by covalent modification of critical cysteine residues. Mol Microbiol. 2002;45:243–254. - PubMed
-
- Chiappetta G. Ndiaye S. Igbaria A. Kumar C. Vinh J. Toledano MB. Proteome screens for Cys residues oxidation: the redoxome. Methods Enzymol. 2010;473:199–216. - PubMed
-
- Depuydt M. Messens J. Collet JF. How proteins form disulfide bonds. Antioxid Redox Signal. 2011;15:49–66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

