In vivo imaging implicates CCR2(+) monocytes as regulators of neutrophil recruitment during arthritis
- PMID: 23121982
- PMCID: PMC3760198
- DOI: 10.1016/j.cellimm.2012.07.005
In vivo imaging implicates CCR2(+) monocytes as regulators of neutrophil recruitment during arthritis
Abstract
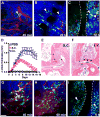
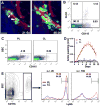
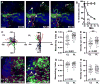
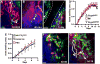
The infiltration of neutrophils and monocytes is a prominent feature of inflammatory diseases including human rheumatoid arthritis. Understanding how neutrophil recruitment is regulated during pathogenesis is crucial for developing anti-inflammatory therapies. We optimized the K/B×N serum-induced mouse arthritis model to study neutrophil trafficking dynamics in vivo using two-photon microscopy. Arthritogenic serum was injected subcutaneously into one hind footpad to induce a local arthritis with robust neutrophil recruitment. Using this approach, we showed that the depletion of monocytes with clodronate liposomes impaired neutrophil recruitment specifically at the transendothelial migration step. The depletion of CCR2(+) monocytes with the monoclonal antibody MC-21 reproduced these effects, implicating CCR2(+) monocytes as key regulators of neutrophil extravasation during arthritis initiation. However, monocyte depletion did not prevent neutrophil extravasation in response to bacterial challenge. These findings suggest that anti-inflammatory therapies targeting monocytes may act in part through antagonizing neutrophil extravasation at sites of aseptic inflammation.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

References
-
- Feldmann M, Brennan FM, Maini RN. Rheumatoid Arthritis. Cell. 1996;85:307–310. - PubMed
-
- Norberg B, Eriksson S. Joint fluid leukocytosis of patients with rheumatoid arthritis evidence for neutrophil and monocyte chemotaxis in vivo. Clin Rheumatol. 1983;2:237–242. - PubMed
-
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. - PubMed
-
- Edwards SW, Hallett MB. Seeing the wood for the trees: the forgotten role of neutrophils in rheumatoid arthritis. Immunol Today. 1997;18:320–324. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

