Brainstem nutrient sensing in the nucleus of the solitary tract inhibits feeding
- PMID: 23123165
- PMCID: PMC3537851
- DOI: 10.1016/j.cmet.2012.10.003
Brainstem nutrient sensing in the nucleus of the solitary tract inhibits feeding
Abstract
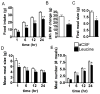
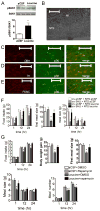
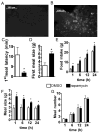
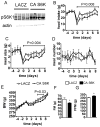
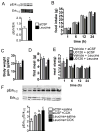
Direct detection of circulating nutrients by the central nervous system has been implicated in the regulation of energy balance, and the mediobasal hypothalamus is considered as the primary sensing site mediating these effects. Neurons sensitive to energyrelated signals have also been identified outside the hypothalamus, particularly within the caudomedial nucleus of the solitary tract (cmNTS) in brainstem, but the consequences of direct cmNTS nutrient detection on energy balance remain poorly characterized. Here we determined the behavioral and metabolic consequences of direct L-leucine detection by the cmNTS and investigated the intracellular signaling and neurochemical pathways implicated in cmNTS L-leucine sensing in rats. Our results support the distributed nature of central nutrient detection, evidence a role for the cmNTS S6K1 pathway in the regulation of meal size and body weight, and suggest that the cmNTS integrates direct cmNTS nutrient detection with gut-derived, descending forebrain, and adiposity signals of energy availability to regulate food intake.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
References
-
- Azzara AV, Sokolnicki JP, Schwartz GJ. Central melanocortin receptor agonist reduces spontaneous and scheduled meal size but does not augment duodenal preload-induced feeding inhibition. Physiol Behav. 2002;77:411–416. - PubMed
-
- Blevins JE, Schwartz MW, Baskin DG. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol. 2004;287:R87–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

