Inhibition of the Jak-STAT pathway prevents CNTF-mediated survival of axotomized oxytocinergic magnocellular neurons in organotypic cultures of the rat supraoptic nucleus
- PMID: 23123407
- PMCID: PMC3552118
- DOI: 10.1016/j.expneurol.2012.10.023
Inhibition of the Jak-STAT pathway prevents CNTF-mediated survival of axotomized oxytocinergic magnocellular neurons in organotypic cultures of the rat supraoptic nucleus
Abstract
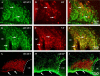
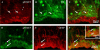
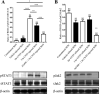
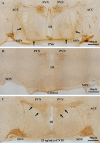
Previous studies have demonstrated that ciliary neurotrophic factor (CNTF) enhances survival and process outgrowth from magnocellular neurons in the paraventricular (PVN) and the supraoptic (SON) nuclei. However, the mechanisms by which CNTF facilitates these processes remain to be determined. Therefore, the aim of this study was to identify the immediate signal transduction events that occur within the rat SON following administration of exogenous rat recombinant CNTF (rrCNTF) and to determine the contribution of those intracellular signaling pathway(s) to neuronal survival and process outgrowth, respectively. Immunohistochemical and Western blot analyses demonstrated that axonal injury and acute unilateral pressure injection of 100 ng/μl of rrCNTF directly over the rat SON resulted in a rapid and transient increase in phosphorylated-STAT3 (pSTAT3) in astrocytes but not neurons in the SON in vivo. Utilizing rat hypothalamic organotypic explant cultures, we then demonstrated that administration of 25 ng/ml rrCNTF for 14days significantly increased the survival and process outgrowth of OT magnocellular neurons. In addition, pharmacological inhibition of the Jak-STAT pathway via AG490 and cucurbitacin I significantly reduced the survival of OT magnocellular neurons in the SON and PVN; however, the contribution of the Jak-STAT pathway to CNTF-mediated process outgrowth remains to be determined. Together, these data indicate that CNTF-induced survival of OT magnocellular neurons is mediated indirectly through astrocytes via the Jak-STAT signaling pathway.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

References
-
- Albrecht PJ, Dahl JP, Stoltzfus OK, Levenson R, Levison SW. Ciliary neurotrophic factor activates spinal cord astrocytes, stimulating their production and release of fibroblast growth factor-2, to increase motor neuron survival. Exp Neurol. 2002;173:46–62. - PubMed
-
- Alonzi T, Middleton G, Wyatt S, Buchman V, Betz UA, Muller W, Musiani P, Poli V, Davies AM. Role of STAT3 and PI 3-kinase/Akt in mediating the survival actions of cytokines on sensory neurons. Mol Cell Neurosci. 2001;18:270–282. - PubMed
-
- Bechstein M, Haussler U, Neef M, Hofmann HD, Kirsch M, Haas CA. CNTF-mediated preactivation of astrocytes attenuates neuronal damage and epileptiform activity in experimental epilepsy. Exp Neurol. 2012;236:141–150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

